(a) In aqueous solution, persulfate ions, \(S_2O_8^{2–}\), react with iodide ions, as shown in reaction 1.
![]()
The rate of reaction 1 is investigated. A sample of \(S_2O_8^{2–}\) is mixed with a large excess of iodide ions of known concentration. The graph in Fig. 5.1 shows the results obtained.
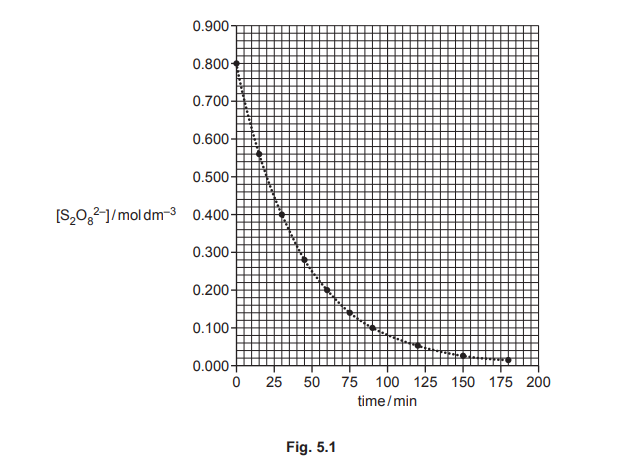
(i) Use Fig. 5.1 to determine the initial rate of reaction 1. Show your working.
(ii) The rate equation for reaction 1 is rate = \(k [S_2O_8^{2–}] [I^–]\). Suggest why a large excess of iodide ions allows the rate constant to be determined from the half-life in this investigation.
(b) The reaction of persulfate ions, \(S_2O_8^{2–}\), with iodide ions is catalysed by \(Fe^{2+}\) ions. Write two equations to show how \(Fe^{2+}\) catalyses reaction 1.
(c) Describe the effect of an increase in temperature on the rate constant and the rate of reaction 1.
(d) In aqueous solution, thiosulfate ions, \(S_2O_3^{2–}\), react with hydrogen ions, as shown in reaction 2.
![]()
The rate of reaction is first order with respect to [\(S_2O_3^{2–}\)] and zero order with respect to [\(H^+\)] under certain conditions. The rate constant, k, for this reaction is \(1.58 \times 10^{–2} s^{–1}\). Calculate the half-life, \(t_{\frac{1}{2}}\) , for reaction 2.
(e) The compound nitrosyl bromide, NOBr, can be formed as shown in reaction 3. ![]()
The rate is first order with respect to [NO] and first order with respect to [\(Br_2\)]. The reaction mechanism has two steps. Suggest equations for the two steps of this mechanism. State which is the rate-determining step.
▶️ Answer/Explanation
(a)(i)
Answer: The initial rate is determined from the tangent at \( t = 0 \). From the graph, the slope is approximately \(-0.0125 \, \text{mol dm}^{-3} \, \text{s}^{-1}\).
Explanation: The initial rate is the change in concentration of \( S_2O_8^{2-} \) per unit time at \( t = 0 \). The tangent at \( t = 0 \) gives a slope of \(-0.0125\), so the rate is \( 0.0125 \, \text{mol dm}^{-3} \, \text{s}^{-1} \).
(a)(ii)
Answer: A large excess of \( I^- \) ensures its concentration remains approximately constant, making the reaction pseudo-first order with respect to \( S_2O_8^{2-} \). The half-life then depends only on \( k \).
Explanation: Since \( [I^-] \) is constant, the rate equation simplifies to \( \text{rate} = k’ [S_2O_8^{2-}] \), where \( k’ = k[I^-] \). The half-life of a first-order reaction is \( t_{1/2} = \frac{\ln 2}{k’} \), allowing \( k \) to be determined.
(b)
Answer: \[ Fe^{2+} + S_2O_8^{2-} \rightarrow Fe^{3+} + 2SO_4^{2-} \] \[ Fe^{3+} + I^- \rightarrow Fe^{2+} + \frac{1}{2} I_2 \]
Explanation: \( Fe^{2+} \) is oxidised to \( Fe^{3+} \) by \( S_2O_8^{2-} \), and \( Fe^{3+} \) is reduced back to \( Fe^{2+} \) by \( I^- \), regenerating the catalyst.
(c)
Answer: An increase in temperature increases the rate constant \( k \) (Arrhenius equation) and thus the rate of reaction.
Explanation: The Arrhenius equation \( k = Ae^{-E_a/RT} \) shows that \( k \) increases with temperature. Since rate \( = k[S_2O_8^{2-}][I^-] \), the rate also increases.
(d)
Answer: The half-life \( t_{1/2} = \frac{\ln 2}{k} = \frac{0.693}{1.58 \times 10^{-2}} \approx 43.9 \, \text{s} \).
Explanation: For a first-order reaction, \( t_{1/2} = \frac{\ln 2}{k} \). Substituting \( k = 1.58 \times 10^{-2} \, \text{s}^{-1} \) gives \( t_{1/2} \approx 43.9 \, \text{s} \).
(e)
Answer: Step 1 (slow, rate-determining): \( NO + Br_2 \rightarrow NOBr_2 \)
Step 2 (fast): \( NOBr_2 + NO \rightarrow 2NOBr \)
Explanation: The rate-determining step involves one NO and one \( Br_2 \) molecule, matching the observed rate law. The second step is fast and does not affect the rate.