(a) The pH of a saturated solution of calcium hydroxide is 12.35 at 298K.
(i) Show that the concentration of hydroxide ions in a saturated solution of calcium hydroxide is 0.0224 moldm⁻³ at 298K.
(ii) Use data given in (i) to calculate the solubility product, \(K_{sp}\), of calcium hydroxide at 298K. Include the units of \(K_{sp}\) in your answer.
(iii) A spatula measure of solid calcium chloride is stirred into a sample of saturated calcium hydroxide solution. All of the calcium chloride dissolves. Describe one other observation that would be made and give an estimated value of the pH of the solution obtained. Explain both your answers.
(iv) Calcium hydroxide reacts with dilute sulfuric acid to form calcium sulfate. Barium hydroxide behaves in a similar way, forming barium sulfate. Explain why calcium sulfate is more soluble in water than barium sulfate.
(b) Some solid calcium is added to an excess of aqueous ethanoic acid, CH₃COOH, and left until all the calcium has reacted. The resulting mixture, mixture D, contains no undissolved solids.
(i) Write an equation for the reaction of calcium with CH₃COOH.
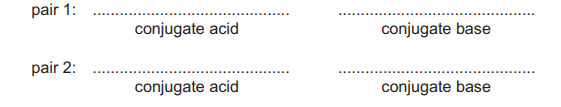
(ii) Use formulae of molecules and ions to identify two conjugate acid–base pairs present in mixture D.
Pair 1 should consist of organic species.
(iii) Write the expression for the \(K_a\) of CH₃COOH.
(iv) The concentration of calcium ethanoate, (CH₃COO)₂Ca, in mixture D is 0.394 moldm⁻³. The concentration of CH₃COOH in mixture D is 0.270 moldm⁻³. The \(K_a\) of CH₃COOH is 1.74 × 10⁻⁵ moldm⁻³ at 298K. Calculate the pH of mixture D.
(v) Write two equations to show how mixture D can act as a buffer solution.
▶️ Answer/Explanation
(a)(i) \([H^+] = 10^{–12.35} = 4.47 \times 10^{–13} \text{ moldm}^{-3}\)
Using \(K_w = [H^+][OH^-]\), \([OH^-] = \frac{1.0 \times 10^{-14}}{4.47 \times 10^{-13}} = 0.0224 \text{ moldm}^{-3}\).
Explanation: The hydroxide ion concentration is derived from the pH using the ionic product of water, \(K_w\).
(a)(ii) \(K_{sp} = [Ca^{2+}][OH^-]^2 = (0.0112)(0.0224)^2 = 5.62 \times 10^{-6} \text{ mol}^3 \text{dm}^{-9}\).
Explanation: The solubility product is calculated using the equilibrium concentrations of calcium and hydroxide ions.
(a)(iii) Observation: White precipitate forms.
pH of solution: ~12 (slightly lower than 12.35).
Explanation: The common ion effect reduces the solubility of calcium hydroxide, lowering pH slightly.
(a)(iv) Calcium sulfate is more soluble because its hydration energy outweighs its lattice energy more significantly than for barium sulfate.
Explanation: The smaller size of Ca²⁺ leads to stronger hydration, increasing solubility.
(b)(i) \(\text{Ca} + 2\text{CH}_3\text{COOH} \rightarrow \text{Ca(CH}_3\text{COO)}_2 + \text{H}_2\).
(b)(ii) Pair 1: \(\text{CH}_3\text{COOH}\) (acid) and \(\text{CH}_3\text{COO}^-\) (base).
Pair 2: \(\text{H}_3\text{O}^+\) (acid) and \(\text{H}_2\text{O}\) (base).
(b)(iii) \(K_a = \frac{[\text{H}^+][\text{CH}_3\text{COO}^-]}{[\text{CH}_3\text{COOH}]}\).
(b)(iv) \([\text{CH}_3\text{COO}^-] = 0.788 \text{ moldm}^{-3}\), \([\text{CH}_3\text{COOH}] = 0.270 \text{ moldm}^{-3}\).
Using \(K_a\), \([\text{H}^+] = 1.74 \times 10^{-5} \times \frac{0.270}{0.788} = 5.96 \times 10^{-6} \text{ moldm}^{-3}\).
\(\text{pH} = -\log(5.96 \times 10^{-6}) = 5.22\).
Explanation: The pH is calculated using the Henderson-Hasselbalch approximation for the buffer system.
(b)(v) Equation 1: \(\text{CH}_3\text{COO}^- + \text{H}^+ \rightarrow \text{CH}_3\text{COOH}\).
Equation 2: \(\text{CH}_3\text{COOH} + \text{OH}^- \rightarrow \text{CH}_3\text{COO}^- + \text{H}_2\text{O}\).
Explanation: These equations show how the buffer resists changes in pH by neutralizing added acids or bases.
