Transition metal atoms and transition metal ions form complexes by combining with ligands.
(a) Explain why transition elements form complex ions.
(b) \(Co^{2+}\) ions form complex ion G. Each G ion contains two \(Co^{2+}\) ions, both of which are octahedrally coordinated. Each G ion contains one \(O_2\) molecule, which donates one pair of electrons to each \(Co^{2+}\) ion, and one \(NH_2^–\) ion, which donates one pair of electrons to each \(Co^{2+}\) ion. The remaining ligands are NH₃ molecules.
(i) Deduce the formula of complex ion G. Include its overall charge.
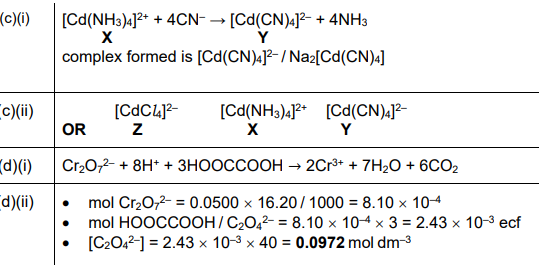
(ii) The d-orbitals of the \(Co^{2+}\) ions present in complex ion G are split. State the number of d-orbitals that are at a higher energy level and the number of d-orbitals that are at a lower energy level in each \(Co^{2+}\) ion.
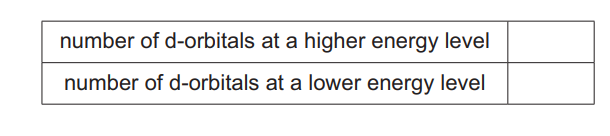
(iii) \(Co^{2+}\) ions form a different complex ion, M. Each M ion contains two \(Co^{2+}\) ions, both of which are octahedrally coordinated, but the ligands are different from the ligands in G. Explain why G and M have different colours.
(c) Cadmium forms complex ion X, \([Cd(NH_3)_4]^{2+}\). When a solution containing CN⁻ ions is added to an aqueous solution of X, a ligand exchange reaction takes place, forming complex ion Y. Y contains no \(NH_3\) ligands and no \(H_2O\) ligands. Y is in a much higher concentration in the mixture than X. The oxidation state and coordination number of cadmium do not change in this reaction.
(i) Write an ionic equation for this reaction, using the formulae of the complex ions.
(ii) Cadmium forms complex ion Z in the same oxidation state and with the same coordination number as in X. All the ligands in Z are Cl⁻ ions. When NaCl(aq) is added to a solution of X, very little Z forms. Write the three cadmium complexes, X, Y and Z, in order of increasing stability constant, \(K_{stab}\).

(d) Ethanedioate ions, \(C_2O_4^{2–}\), form complexes with transition element ions. The concentration of \(C_2O_4^{2–}\) ions can be found by reaction with acidified \(Cr_2O_7^{2–}\) ions. \(C_2O_4^{2–}\) ions are protonated and form HOOCCOOH molecules which are oxidised by \(Cr_2O_7^{2–}\). The half-equations are shown. 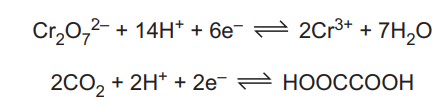
(i) Construct an equation for the reaction between acidified Cr₂O₇²⁻ and HOOCCOOH
(ii) A 25.0 cm³ sample of a solution of Na₂C₂O₄ reacts with exactly 16.20 cm³ of an acidified solution of 0.0500 moldm⁻³ K₂Cr₂O₇. Calculate the concentration of the solution of Na₂C₂O₄.
▶️ Answer/Explanation
(a) Transition elements form complex ions because they have energetically accessible empty d-orbitals that can accept lone pairs of electrons from ligands, forming coordinate (dative) bonds.
Explanation: The partially filled d-subshell in transition metals allows them to form stable complexes with ligands by accepting electron pairs into their d-orbitals.
(b)(i) The formula of complex ion G is \([Co_2O_2NH_2(NH_3)_8]^{3+}\).
Explanation: Each \(Co^{2+}\) is octahedrally coordinated (6 ligands). The \(O_2\) and \(NH_2^-\) each donate one pair to both Co ions (total 4 ligands), and the remaining 8 NH₃ ligands complete the coordination (4 NH₃ per Co). The overall charge is calculated as: \(2(Co^{2+}) + 1(O_2) + 1(NH_2^-) + 8(NH_3) = +3\).
(b)(ii) Higher energy d-orbitals: 2 (\(e_g\) orbitals). Lower energy d-orbitals: 3 (\(t_{2g}\) orbitals).
Explanation: In octahedral complexes, d-orbitals split into two higher energy \(e_g\) orbitals (dz² and dx²-y²) and three lower energy \(t_{2g}\) orbitals (dxy, dxz, dyz).
(b)(iii) G and M have different colors because the energy gap (\(\Delta E\)) between split d-orbitals differs due to different ligand field strengths.
Explanation: The color depends on the energy gap between d-orbitals. Different ligands (in G vs M) cause different splitting patterns, absorbing different wavelengths of light.
(c)(i) \([Cd(NH_3)_4]^{2+} + 4CN^- \rightarrow [Cd(CN)_4]^{2-} + 4NH_3\)
Explanation: CN⁻ ligands replace NH₃ in the complex, forming the more stable \([Cd(CN)_4]^{2-}\) complex.
(c)(ii) Order of increasing stability constant: \([CdCl_4]^{2-} < [Cd(NH_3)_4]^{2+} < [Cd(CN)_4]^{2-}\)
Explanation: CN⁻ forms the most stable complex (highest \(K_{stab}\)) due to strong field strength, while Cl⁻ forms the least stable complex.
(d)(i) \(Cr_2O_7^{2-} + 3HOOCCOOH + 8H^+ \rightarrow 2Cr^{3+} + 6CO_2 + 7H_2O\)
Explanation: The balanced equation combines the half-reactions: Cr₂O₇²⁻ is reduced to Cr³⁺ (6 electrons gained), while HOOCCOOH is oxidized to CO₂ (2 electrons lost per molecule).
(d)(ii) The concentration of Na₂C₂O₄ is 0.0972 moldm⁻³.
Calculation:
• Moles of Cr₂O₇²⁻ = 0.0500 × 0.01620 = 0.00081 mol
• Moles of C₂O₄²⁻ = 3 × 0.00081 = 0.00243 mol (1:3 stoichiometry)
• Concentration = 0.00243 mol / 0.0250 dm³ = 0.0972 moldm⁻³