(a) (i) Define transition element.
(ii) Explain why transition elements can form complex ions.
(b) The 3d orbitals in an isolated Ag⁺ ion are degenerate.
(i) Define degenerate d orbitals.
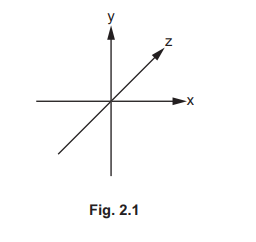
(ii) Sketch the shape of a 3dxy orbital in Fig. 2.1.
(c) Tollens’ reagent can be used to distinguish between aldehydes and ketones. Tollens’ reagent contains [Ag(NH₃)₂]OH, which can be prepared in a two-step process.
step 1 Aqueous NaOH is added dropwise to aqueous AgNO₃ to form Ag₂O as a brown precipitate.
step 2 Aqueous NH₃ is added dropwise to Ag₂O to form a colourless solution containing [Ag(NH₃)₂]OH.
Construct equations for each of the steps in the preparation of [Ag(NH₃)₂]OH.
(d) Name the shape of the complex ion \([Ag(NH_3)_2]^+\). State the bond angle for H-N-Ag and for N-Ag-N.
(e) An electrochemical cell uses Ag₂O as the positive electrode and Zn as the negative electrode immersed in an alkaline electrolyte.
The overall cell reaction is shown.
Ag₂O + Zn + H₂O → 2Ag + Zn(OH)₂
Complete the half-equation for the reaction at each electrode.
at the positive electrode Ag₂O + …………
at the negative electrode Zn + …………..
(f) Coordination polymers are made when a bidentate ligand acts as a bridge between different metal ions. Under certain conditions \(Ru^{3+}\)(aq) and the bidentate ligand dps can form a coordination polymer containing \(([Ru(dps)Cl_4]^–)_n\) chains.
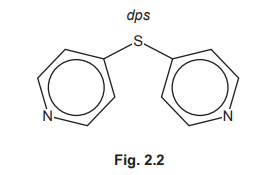
The bidentate ligand dps uses each of the nitrogen atoms to bond to a different \(Ru^{3+}\). Complete Fig. 2.3 by drawing the structure for the coordination polymer \(([Ru(dps)Cl_4]^–)_n\). Show two repeat units. The dps ligand can be represented using 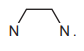
▶️ Answer/Explanation
(a)(i) A transition element is an element that forms one or more stable ions with incomplete d orbitals.
Explanation: Transition elements are characterized by their ability to form ions with partially filled d subshells, such as Fe²⁺ (3d⁶) or Cu²⁺ (3d⁹).
(a)(ii) Transition elements can form complex ions because they have vacant d orbitals that are energetically accessible for bonding with lone pairs from ligands.
Explanation: The presence of empty d orbitals allows transition metals to accept electron pairs from ligands, forming coordinate bonds in complex ions like \([Fe(CN)_6]^{4-}\).
(b)(i) Degenerate d orbitals are orbitals of the same energy level.
Explanation: In an isolated Ag⁺ ion, all five 3d orbitals (dxy, dxz, dyz, dx²-y², dz²) have identical energy.
(b)(ii)
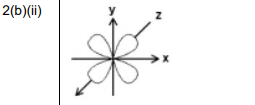
Explanation: The 3dxy orbital has four lobes lying in the xy plane between the axes, with a node at the nucleus.
(c)
Step 1: \(2AgNO_3 + 2NaOH → Ag_2O + 2NaNO_3 + H_2O\)
Step 2: \(Ag_2O + 4NH_3 + H_2O → 2[Ag(NH_3)_2]OH\)
Explanation: In step 1, silver oxide precipitates, while in step 2, it dissolves in ammonia to form the diamminesilver(I) complex.
(d) Linear; H-N-Ag angle: 180°; N-Ag-N angle: 180°.
Explanation: The \([Ag(NH_3)_2]^+\) ion has two ammonia ligands arranged linearly around the central Ag⁺ ion, giving bond angles of 180°.
(e)
Positive electrode: \(Ag_2O + H_2O + 2e^- → 2Ag + 2OH^-\)
Negative electrode: \(Zn + 2OH^- → Zn(OH)_2 + 2e^-\)
Explanation: At the positive electrode, Ag₂O is reduced to Ag, while at the negative electrode, Zn is oxidized to Zn(OH)₂.
(f)
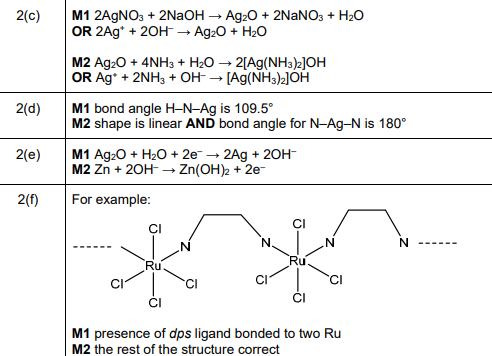
Explanation: The dps ligand bridges between Ru³⁺ ions, with each nitrogen coordinating to a different metal center, forming a polymeric chain with \([Ru(dps)Cl_4]^-\) repeating units.
