The shapes of four different complexes, P, Q, R and S, are shown in Table 5.1. The symbol J represents an atom or ion of a transition element. The symbol L is used to represent a monodentate ligand.
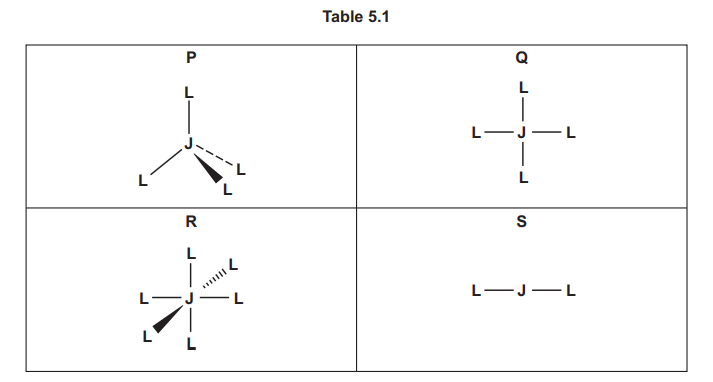
(a) Label one bond angle on each of complexes P, Q, R and S, and identify the size of the angle in degrees.
(b) Identify the shapes of complexes P, Q, R and S.
(c) Two L ligands are exchanged with two different monodentate ligands X and Y in each of complexes P, Q, R and S. Identify all the complexes which form new complexes that show geometrical isomerism.
(d) Three L ligands are exchanged with three different monodentate ligands X, Y and Z in each of complexes P, Q and R. Identify all the complexes which form new complexes that show optical isomerism.
▶️ Answer/Explanation
(a) Bond Angles:
- P: 109.5° (tetrahedral angle between any two bonds)
- Q: 90° (square planar angle between adjacent ligands)
- R: 90° (octahedral angle between adjacent ligands)
- S: 180° (linear angle between the two ligands)
Explanation: The bond angles are determined by the geometry of each complex. Tetrahedral complexes have 109.5° angles, square planar and octahedral complexes have 90° angles between adjacent ligands, and linear complexes have 180° angles.
(b) Shapes:
- P: Tetrahedral (4 ligands around central atom J)
- Q: Square planar (4 ligands in a plane around J)
- R: Octahedral (6 ligands around J)
- S: Linear (2 ligands around J)
Explanation: The shapes are identified based on the coordination number and arrangement of ligands. P has 4 ligands (tetrahedral), Q has 4 ligands in a plane (square planar), R has 6 ligands (octahedral), and S has 2 ligands (linear).
(c) Complexes showing geometrical isomerism: Q and R.
Explanation: Geometrical isomerism (cis/trans) occurs in square planar (Q) and octahedral (R) complexes when two different ligands (X and Y) replace two L ligands. Tetrahedral (P) and linear (S) complexes cannot exhibit geometrical isomerism due to their symmetrical arrangements.
(d) Complexes showing optical isomerism: P and R.
Explanation: Optical isomerism arises when a complex is chiral (non-superimposable on its mirror image). Tetrahedral (P) and octahedral (R) complexes with three different ligands (X, Y, Z) can form chiral structures. Square planar (Q) complexes do not typically show optical isomerism.
