Transition metal atoms and transition metal ions form complexes by combining with species called ligands.
(a) When NaOH(aq) is added to an aqueous solution containing \([Co(H_2O)_6]^{2+}\) a precipitation reaction occurs accompanied by a colour change. In this reaction, two of the water ligands each lose one H⁺ ion. The H⁺ ions are gained by OH⁻ ions from the NaOH(aq).
(i) State the colour change seen in this precipitation reaction.
(ii) Complete the ionic equation for this precipitation reaction.
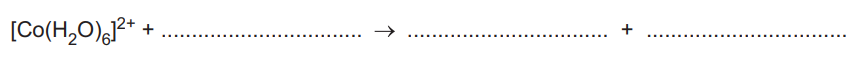
(iii) This precipitation reaction can also be described as a different type of reaction. Name this type of reaction.
(b) L is an uncharged tridentate ligand. L donates three lone pairs to a metal atom or ion. Cobalt forms an octahedral complex ion, E, with L. Complex ion E has a 2+ charge.
(i) Give the formula of E.
(ii) Identify the oxidation state of cobalt in E.
(iii) The d-orbitals of the cobalt atom or ion present in E are split in energy. State the number of d-orbitals that are at a higher energy level and the number of d-orbitals that are at a lower energy level.

(iv) Define the term non-degenerate d-orbitals.
(c) The mineral chromite contains a compound which has the formula \(FeCr_nO_4\). The oxidation state of iron in \(FeCr_nO_4\) is +2. A sample of 4.18g of \(FeCr_nO_4\) is dissolved in an excess of sulfuric acid. The resulting solution is made up to 250 cm³. This is solution F. All the Fe²⁺ ions in 25.0 cm³ of solution F are oxidised to Fe³⁺ ions by exactly 18.7 cm³ of 0.0200 mol/dm³ KMnO₄. One \(MnO_4^–\) ion reacts with five Fe²⁺ ions. Assume no other oxidation reaction occurs.
(i) Write an equation for the reaction of Fe²⁺ ions with \(MnO_4^–\) ions in acid solution.
(ii) Calculate the number of moles of Fe²⁺ ions in 25.0 cm³ of solution F.
(iii) Calculate the Mr of \(FeCr_nO_4\) and use your answer to deduce the value of n.
▶️ Answer/Explanation
(a)(i) From red/pink to blue.
Explanation: The colour change occurs as \([Co(H_2O)_6]^{2+}\) (pink) reacts with \(OH^–\) to form \(Co(H_2O)_4(OH)_2\) (blue precipitate).
(a)(ii) \([Co(H_2O)_6]^{2+} + 2OH^– \to Co(H_2O)_4(OH)_2 + 2H_2O\).
Explanation: The equation shows the replacement of two \(H_2O\) ligands by \(OH^–\) ions, forming a neutral precipitate.
(a)(iii) Acid-base reaction.
Explanation: The transfer of \(H^+\) from \(H_2O\) ligands to \(OH^–\) classifies this as an acid-base reaction.
(b)(i) \([CoL_2]^{2+}\).
Explanation: Each tridentate ligand (L) occupies 3 coordination sites, so 2 ligands complete the octahedral geometry around Co²⁺.
(b)(ii) Oxidation state of cobalt: +2.
Explanation: The complex has a 2+ charge, and L is neutral, so Co must be in the +2 oxidation state.
(b)(iii) Higher energy: 2, Lower energy: 3.
Explanation: In an octahedral field, d-orbitals split into 2 higher-energy \(e_g\) orbitals and 3 lower-energy \(t_{2g}\) orbitals.
(b)(iv) Non-degenerate d-orbitals have different energy levels.
Explanation: Degenerate orbitals have the same energy; splitting in a ligand field removes this degeneracy.
(c)(i) \(5Fe^{2+} + MnO_4^– + 8H^+ \to 5Fe^{3+} + Mn^{2+} + 4H_2O\).
Explanation: The balanced redox equation shows the oxidation of Fe²⁺ by \(MnO_4^–\) in acidic medium.
(c)(ii) Moles of Fe²⁺: 1.87 × 10⁻³.
Explanation: Moles of \(MnO_4^–\) = 0.0200 × 0.0187 = 3.74 × 10⁻⁴. Since 1 \(MnO_4^–\) reacts with 5 Fe²⁺, moles of Fe²⁺ = 5 × 3.74 × 10⁻⁴ = 1.87 × 10⁻³.
(c)(iii) \(M_r = 224\), n = 2.
Explanation:
– Total moles of Fe²⁺ in 250 cm³ = 1.87 × 10⁻³ × 10 = 1.87 × 10⁻².
– \(M_r = \frac{4.18}{1.87 \times 10^{-2}} = 224\).
– For \(FeCr_nO_4\): 56 + 52n + 64 = 224 → \(n = 2\).
