(a) Describe the trend in the solubility of the sulfates of magnesium, calcium and strontium. Explain your answer.

(b) Define lattice energy, \(\Delta H_{latt}\).
(c) State and explain the main factors that affect the magnitude of lattice energies.
(d) Table 1.1 shows some energy changes.
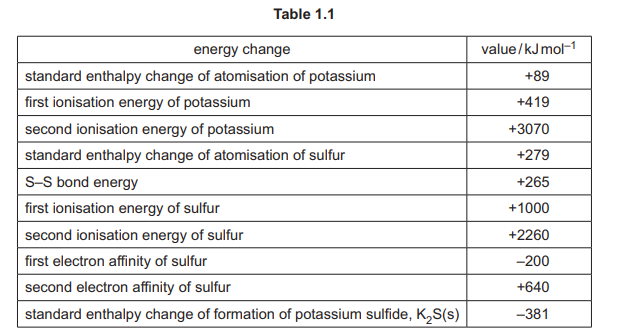
(i) Born–Haber cycles can be used to determine the lattice energies of ionic compounds. Complete the Born–Haber cycle in Fig. 1.1 for potassium sulfide, \(K_2S\)(s). Include state symbols for all of the species.
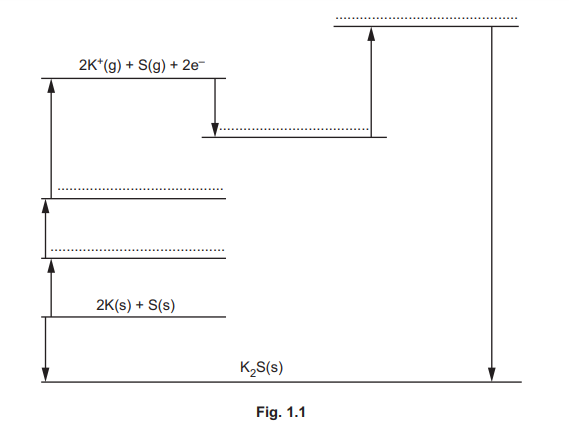
(ii) Calculate the lattice energy, \(\Delta H^{\Theta}_{latt}\), of \(K_2S\)(s) using relevant data from Table 1.1. Show your working.
▶️ Answer/Explanation
(a)
Explanation: The solubility trend is magnesium > calcium > strontium. This is because both lattice energy (\(\Delta H_{latt}\)) and hydration energy (\(\Delta H_{hyd}\)) become less exothermic down the group. The hydration energy dominates, making the overall \(\Delta H_{sol}\) less exothermic (or more endothermic) for larger ions.
(b)
Explanation: Lattice energy (\(\Delta H_{latt}\)) is the energy change when 1 mole of an ionic solid is formed from its gaseous ions under standard conditions.
(c)
Explanation: The magnitude of lattice energy depends on (1) ionic radii (larger ions → less exothermic \(\Delta H_{latt}\)) and (2) ionic charge (higher charge → more exothermic \(\Delta H_{latt}\)).
(d)(i)

Explanation: The Born-Haber cycle includes steps for atomization, ionization, electron affinity, and formation of \(K_2S\)(s) from its elements.
(d)(ii)
Explanation: Using Hess’s Law, the lattice energy is calculated as follows:
\(\Delta H_f^\Theta = 2 \times \Delta H_{atom}(K) + 2 \times IE(K) + \Delta H_{atom}(S) + EA(S) + \Delta H_{latt}\)
Substituting values: \(-381 = (2 \times 89) + (2 \times 419) + 279 + (-200) + 640 + \Delta H_{latt}\)
Solving gives \(\Delta H_{latt}^\Theta = -2116 \text{ kJ mol}^{-1}\).
