(a) Samples of phenol, C₆H₅OH, are reacted separately with sodium and with dilute nitric acid.
(i) Write the equation for the reaction of C₆H₅OH with Na.
(ii) Draw the structures of the two major isomeric organic products formed in the reaction of phenol with dilute HNO₃.
(b) Salicylic acid can be synthesised from phenol.
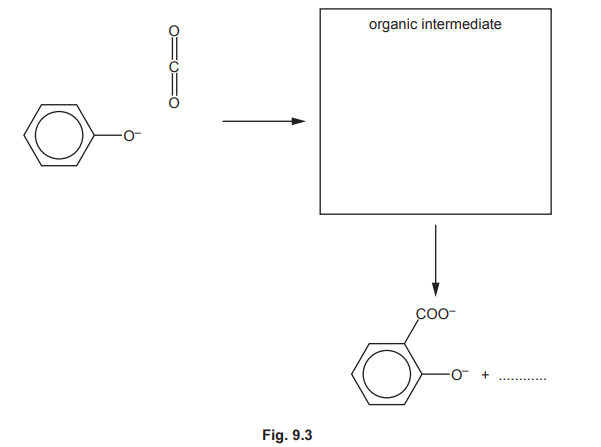
One of the steps in this synthesis is the electrophilic substitution reaction of carbon dioxide with the phenoxide ion, C₆H₅O⁻. Complete the mechanism in Fig. 9.3 for the reaction of C₆H₅O⁻ with \(CO_2\). Include all relevant curly arrows, dipoles and charges. Draw the structure of the organic intermediate.
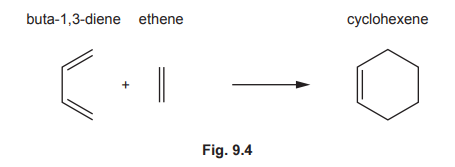
(c) Some syntheses use Diels–Alder reactions, which normally involve a diene and an alkene reacting together to form a cyclohexene.
(i) Draw three curly arrows in Fig. 9.4 to complete the mechanism for the Diels–Alder reaction between buta-1,3-diene and ethene.
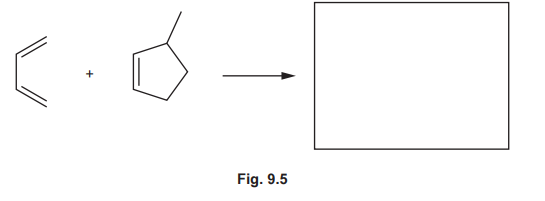
(ii) Another Diels–Alder reaction of buta-1,3-diene is shown in Fig. 9.5. Predict the product formed in this reaction.
▶️ Answer/Explanation
(a)(i) \(2C_6H_5OH + 2Na \rightarrow 2C_6H_5O^-Na^+ + H_2\)
Explanation: Phenol reacts with sodium to form sodium phenoxide (\(C_6H_5O^-Na^+\)) and hydrogen gas, similar to alcohols.
(a)(ii) Major products: 2-nitrophenol and 4-nitrophenol.
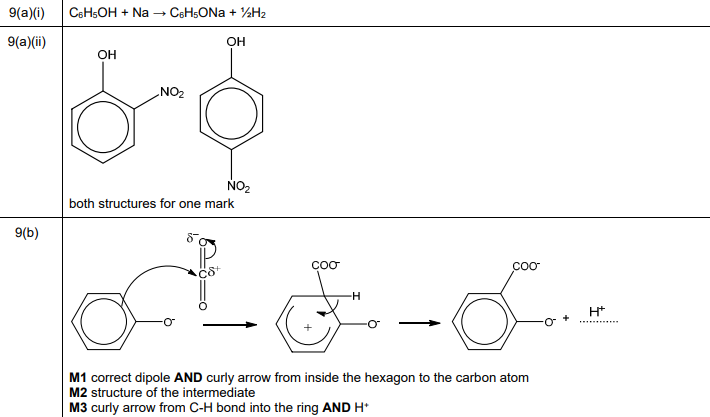
Explanation: Nitration of phenol with dilute HNO₃ preferentially substitutes at the ortho (2-) and para (4-) positions due to the \(-OH\) group’s activating and directing effects.
(b) Mechanism for reaction of phenoxide ion (\(C_6H_5O^-\)) with \(CO_2\):
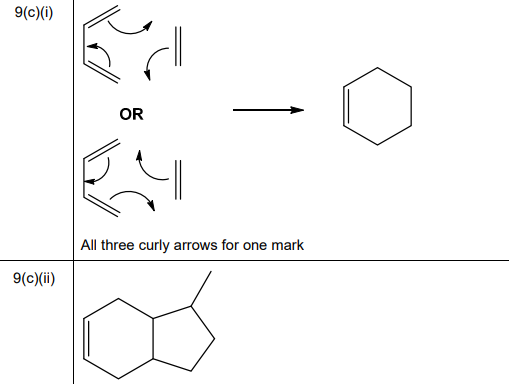
Explanation:
- Nucleophilic attack by phenoxide on the electrophilic carbon of \(CO_2\).
- Formation of a carboxylate intermediate (salicylate ion).
- Protonation yields salicylic acid.
(c)(i) Diels-Alder mechanism (buta-1,3-diene + ethene):

Explanation: Three curly arrows show the concerted formation of two new σ-bonds and one π-bond, resulting in cyclohexene.
(c)(ii) Product of buta-1,3-diene with maleic anhydride:

Explanation: The reaction yields a cyclohexene derivative with an anhydride group, retaining stereochemistry of the dienophile (cis).
