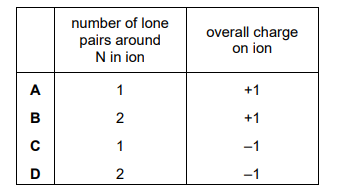
An ion contains 1 nitrogen atom and 2 hydrogen atoms. It has an H–N–H bond angle of approximately 105°. Which row is correct?
▶️ Answer/Explanation
Ans: D
The ion described is \(\text{NH}_2^-\), which has a bent shape due to the lone pair on nitrogen. The bond angle of 105° is consistent with a bent molecular geometry, where the lone pair repels the bonding pairs, reducing the angle from the ideal tetrahedral angle of 109.5°. The correct row in the table is D, as it matches the ion’s formula, shape, and bond angle.
This question is about the first ionisation energies of magnesium and neon. Which row is correct?

▶️ Answer/Explanation
Ans: D
Neon has a higher first ionisation energy than magnesium because it is a noble gas with a full valence shell, making it more stable. Magnesium’s first ionisation energy is lower as it readily loses one electron to achieve stability. The equation for first ionisation energy is: \( X(g) \rightarrow X^+(g) + e^- \). Thus, the correct row is D, where neon’s ionisation energy is greater than magnesium’s.
The first eight successive ionisation energies for two elements of Period 3 of the Periodic Table are shown in the graphs.

What is the formula of the ionic compound formed from these elements?
▶️ Answer/Explanation
Ans: A
The graphs show two elements from Period 3. The first element has a large jump after the second ionisation energy, indicating it is Mg (Group 2). The second element has a large jump after the first ionisation energy, indicating it is Cl (Group 17). The ionic compound formed is MgCl₂, where Mg loses 2 electrons and Cl gains 1 electron each, balancing the charges.
What is the density of a sample of fluorine gas at \(32^\circ C\) and 100 000Pa? Assume fluorine behaves as an ideal gas under these conditions.
▶️ Answer/Explanation
Ans: B
Using the ideal gas law \( pV = nRT \) and the relationship \( \rho = \frac{m}{V} \), we derive the density formula for gases: \( \rho = \frac{pM}{RT} \). For fluorine gas (\( F_2 \)) with molar mass \( M = 38.00 \, \text{g mol}^{-1} \), at \( T = 32^\circ C = 305.15 \, \text{K} \) and \( p = 100\,000 \, \text{Pa} \), the calculation gives:
\[ \rho = \frac{(100\,000)(38.00)}{(8.314)(305.15)} \approx 1.5 \, \text{g dm}^{-3} \]
Thus, the correct answer is B.
