Question
Elements X, Y and Z are all in the first two periods of the Periodic Table.
Their Pauling electronegativity values, \(E_N\), are shown.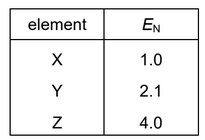
Substances exist with formulae XZ, YZ and \(Z_2\).
Which row puts these substances in order of increasing melting point?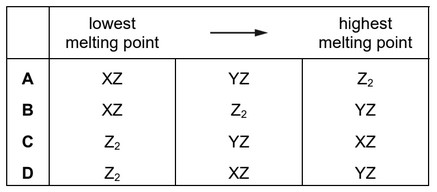
Answer/Explanation
Ans: C
Question
Which type of interaction exists between water molecules and metal cations in aqueous solution?
A dipole-dipole interactions
B hydrogen bonds
C ion-dipole interactions
D ionic bonds
Answer/Explanation
Answer C
Question
Liquids X and Y do not react with one another. They have identical boiling points.
When a particular volume of X is shaken with a similar volume of Y, they form a liquid mixture Z. The average intermolecular forces in liquid Z are stronger than the average of the forces in X and the forces in Y.
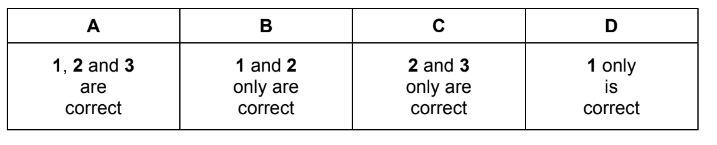
Which deductions from this information are correct?
1 The mixing of X and Y is exothermic.
2 The vapour pressure of liquid Z will be less than that of either liquid X or liquid Y at the same temperature.
3 The boiling point of liquid Z will be lower than that of either liquid X or liquid Y at the same pressure.
▶️Answer/Explanation
Answer B
Question
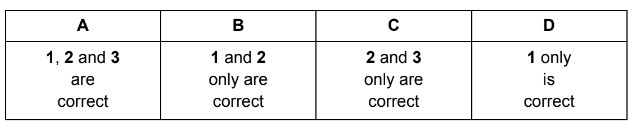
Which statements are correct?
1 The hydrogen bonds in ice are more regularly arranged than in water.
2 The solidification of water to form ice is exothermic.
3 Pure water is less dense than ice.
Answer/Explanation
Answer B
