(a) Write an equation to show the reaction for the standard enthalpy change of formation of \(H_2O\). Include state symbols.
(b) Water is one of the products in the reaction of \(B_2O_3\) and \(NH_3\), as shown in reaction 2.
reaction 2 \(B_2O_3 + 2NH_3 → 2 BN + 3H_2O\)
Table 3.1 shows information about the standard enthalpy change of formation, \(ΔH _f^{\theta}\), of some substances.
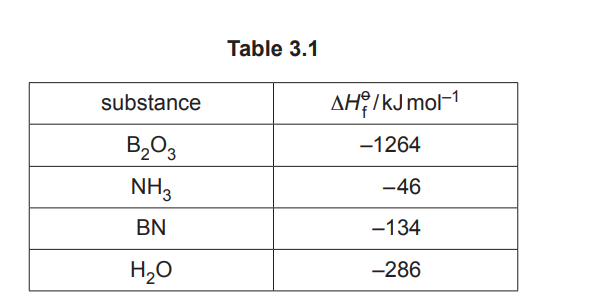
Calculate the enthalpy change, ∆H, for reaction 2 using the data from Table 3.1.
(c) Boron carbide is a hard crystalline solid that has a melting point greater than 2000°C.
(i) Suggest the structure and bonding in boron carbide.
(ii) 100g of pure boron carbide contains 78.26g of boron. Calculate the empirical formula of boron carbide. Show your working.
▶️ Answer/Explanation
(a) \(H_2 (g) + \frac{1}{2}O_2 (g) → H_2O(l)\)
Explanation: The standard enthalpy change of formation (\(\Delta H_f^\theta\)) of \(H_2O\) is defined as the enthalpy change when 1 mole of \(H_2O(l)\) is formed from its elements in their standard states (\(H_2(g)\) and \(O_2(g)\)).
(b) \(\Delta H = +230 \, \text{kJ mol}^{-1}\)
Explanation: Using Hess’s Law, \(\Delta H = \sum \Delta H_f^\theta (\text{products}) – \sum \Delta H_f^\theta (\text{reactants})\):
\(\Delta H = [2 \times (-134) + 3 \times (-286)] – [(-1264) + 2 \times (-46)] = -1074 + 1304 = +230 \, \text{kJ mol}^{-1}\).
(c)(i) Giant covalent structure.
Explanation: Boron carbide’s high melting point (>2000°C) and hardness suggest a giant covalent lattice with strong covalent bonds between boron and carbon atoms.
(c)(ii) Empirical formula: \(B_4C\).
Explanation: – Moles of boron: \(\frac{78.26 \, \text{g}}{10.8 \, \text{g/mol}} = 7.246 \, \text{mol}\).
– Moles of carbon: \(\frac{21.74 \, \text{g}}{12 \, \text{g/mol}} = 1.812 \, \text{mol}\).
– Simplest ratio: \(\frac{7.246}{1.812} \approx 4\) (Boron) : \(1\) (Carbon).
Thus, the empirical formula is \(B_4C\).
