Question
Which types of bonding are present in ammonium carbonate, (NH4)2CO3?
1 ionic
2 covalent
3 co-ordinate (dative covalent)
The responses A to D should be selected on the basis of

Answer/Explanation
Answer:
A
Question
Which statement is correct?
A Ammonia reacts with alkalis to form the ammonium ion.
B Ammonium chloride contains ionic, covalent and co-ordinate bonds.
C The ammonium ion reacts with acids to produce ammonia.
D The bond angle in the ammonium ion is approximately 107°.
Answer/Explanation
Answer B
Question
Which description of the bonding and acid/base nature of aluminium oxide is correct?
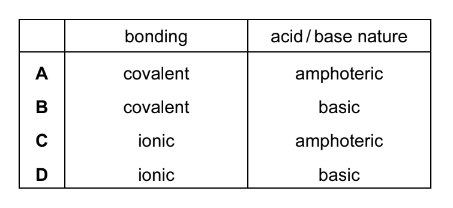
Answer/Explanation
Ans:C
Question
Which types of bonding are present in ammonium carbonate, \((NH_4)2CO_3\)?
1 ionic
2 covalent
3 co-ordinate (dative covalent)
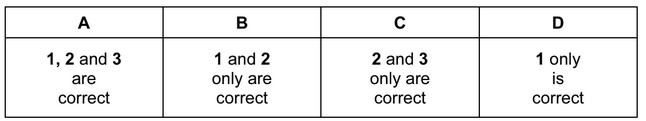
Answer/Explanation
Ans: A
Question
Why is the ionic radius of a chloride ion larger than the ionic radius of a sodium ion?
- A chloride ion has one more occupied electron shell than a sodium ion.
- Chlorine has a higher proton number than sodium.
- Ionic radius increases regularly across the third period.
- Sodium is a metal, chlorine is a non-metal.
Answer/Explanation
Ans:
A
