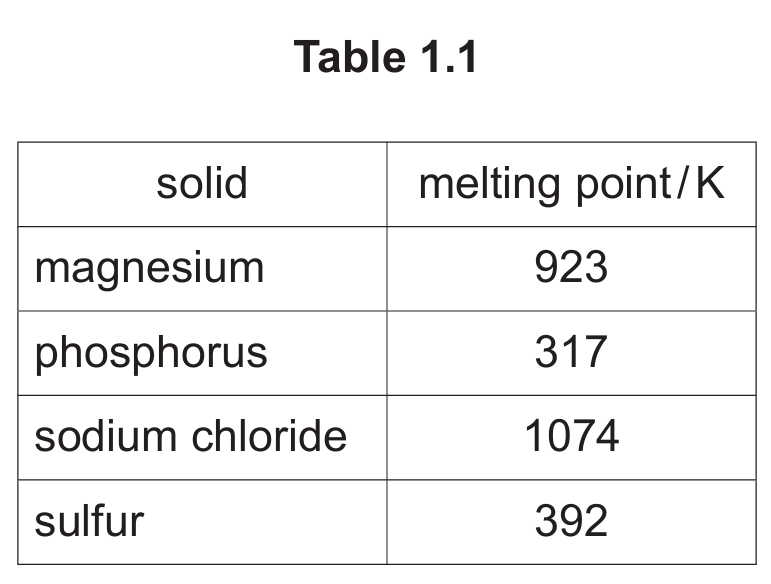
The melting points of some solids are shown in Table 1.1.
(a) (i) State the type of bonding present in magnesium and in sodium chloride.
(a) (ii) Explain the difference in the melting points of magnesium and sodium chloride.
(a) (iii) Explain the difference in the melting points of phosphorus and sulfur in terms of structure and bonding.
(b) (i) Define electronegativity.
(b) (ii) Explain why electronegativity increases across a period.
(b) (iii) Name the strongest intermolecular force that exists between NH3(l) molecules.
(b) (iv) Draw a diagram to show the formation of the strongest intermolecular force between two molecules of NH3(l). Include any relevant lone pairs of electrons and dipoles.
(b) (v) The melting points of ice and ammonia are shown in Table 1.2.
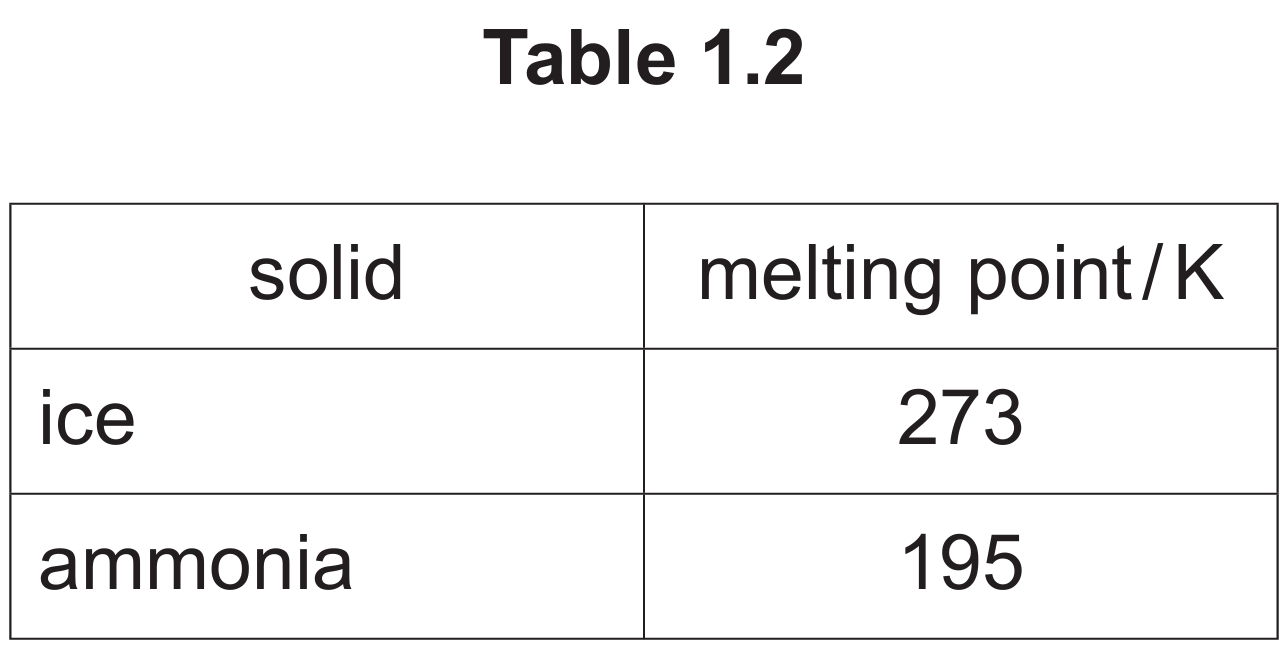
Suggest two reasons for the difference in the melting points of ice and ammonia.
▶️ Answer/Explanation
(a) (i) Bonding in magnesium – metallic; Bonding in sodium chloride – ionic.
Explanation: Magnesium is a metal, so its atoms are held together by a ‘sea of delocalized electrons’ surrounding positive metal ions, which is characteristic of metallic bonding. Sodium chloride is a compound formed from a metal (sodium) and a non-metal (chlorine), resulting in the transfer of electrons and the formation of positive and negative ions held together by strong electrostatic forces, which is ionic bonding.
(a) (ii) Bonds in NaCl are stronger than bonds in Mg.
Explanation: The melting point is a measure of the energy required to overcome the forces holding the particles together in the solid state. Sodium chloride has a higher melting point (1074 K) than magnesium (923 K) because the strong electrostatic forces between the oppositely charged ions (Na⁺ and Cl⁻) in the giant ionic lattice of NaCl are stronger than the metallic bonds between magnesium atoms and the sea of delocalized electrons. While metallic bonds are strong, the ionic bonds in NaCl are even stronger, requiring more energy to break.
(a) (iii) M1: S₈ / molecules of sulfur have more electrons (than P₄ / molecules of phosphorus). M2: S has stronger instantaneous dipole–induced dipole forces (than phosphorus / P).
Explanation: Phosphorus (P₄) and sulfur (S₈) are both simple molecular substances with covalent bonding within the molecules. The forces between these molecules are weak intermolecular forces, specifically instantaneous dipole-induced dipole forces (also known as London dispersion forces). The strength of these forces increases with the number of electrons in a molecule and the surface area. A sulfur molecule (S₈) has more electrons (8 atoms × 16 electrons/atom = 128 electrons) than a phosphorus molecule (P₄) (4 atoms × 15 electrons/atom = 60 electrons). The greater number of electrons in S₈ leads to larger temporary dipoles and stronger instantaneous dipole-induced dipole forces. These stronger intermolecular forces require more energy to overcome during melting, hence sulfur has a higher melting point (392 K) than phosphorus (317 K).
(b) (i) Power of an atom to attract electrons to itself (in a covalent bond).
Explanation: Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is a dimensionless property that is highest for fluorine (the most electronegative element) and decreases down groups and across periods in the periodic table.
(b) (ii) (Across a period)
• Increase in nuclear charge.
• Similar shielding.
• (So) increase in nuclear attraction for bonding / outer / valence electrons OR bonding / outer / valence electron(s) are more strongly attracted to nucleus.
Explanation: Moving from left to right across a period in the periodic table, the number of protons in the nucleus increases. This increases the effective nuclear charge experienced by the outer electrons. Although electrons are being added to the same principal energy shell, the shielding effect by inner electrons remains relatively constant. The combination of increasing nuclear charge and similar shielding means the outer electrons are pulled closer to the nucleus. An atom with a stronger pull on its own electrons will also have a greater power to attract the shared electrons in a covalent bond, hence electronegativity increases across a period.
(b) (iii) Hydrogen bond.
Explanation: Ammonia (NH₃) molecules have a highly polar N-H bond due to the significant difference in electronegativity between nitrogen and hydrogen. The nitrogen atom has a partial negative charge (δ⁻), and the hydrogen atom has a partial positive charge (δ⁺). The strongest intermolecular force between NH₃ molecules is the hydrogen bond, which is a strong dipole-dipole attraction between the lone pair on the nitrogen atom of one molecule and the δ⁺ hydrogen atom of another molecule.
(b) (iv)
M1: Link shown as a dashed line between the lone pair of electrons from N of one NH₃ to one H on other NH₃.
M2: Minimum 3 correct partial charges (on adjacent atoms) over two NH₃ molecules EITHER *N—*H – · · *N OR *H – · · *N—*H.
(A correct diagram would show two NH₃ molecules. One molecule has a lone pair on N. A dashed line is drawn from this lone pair to a H atom on the second molecule. The N atom involved in donating the lone pair is marked δ⁻, the H atom involved is marked δ⁺, and the N atom bonded to that H is also marked δ⁻).
Explanation: A hydrogen bond is a special type of intermolecular force, stronger than van der Waals’ forces but weaker than a covalent bond. It is represented by a dashed or dotted line. It forms when a hydrogen atom, which is covalently bonded to a highly electronegative atom (N, O, or F), experiences attraction to a lone pair of electrons on another highly electronegative atom. The diagram must show the lone pair, the partial charges that create the dipole, and the link between them.
(b) (v)
M1: O is more electronegative than N.
M2: Two H-bonds per water molecule : 1 per ammonia molecule.
Explanation: Ice (solid H₂O) has a higher melting point than solid ammonia (NH₃) because the hydrogen bonding is stronger in water. This is for two main reasons. Firstly, oxygen is more electronegative than nitrogen, meaning the O-H bond in water is more polar than the N-H bond in ammonia. This results in a stronger partial positive charge on the H atom and a stronger partial negative charge on the O atom, leading to a stronger hydrogen bond. Secondly, and crucially, each water molecule can form two hydrogen bonds (using its two H atoms) and accept two more (using the two lone pairs on the oxygen), effectively participating in up to four hydrogen bonds in a highly organized lattice. In contrast, each ammonia molecule can form three hydrogen bonds (using its three H atoms) but can only accept one (using the lone pair on nitrogen). This difference in the number and geometry of possible hydrogen bonds per molecule means the intermolecular forces are collectively stronger in ice, requiring more energy to break them and melt the solid.
