Question
Why does ICl have a higher boiling point than Br₂?
▶️ Answer/Explanation
Solution
Ans: B
ICl has a higher boiling point than Br₂ because it is a polar molecule (due to the electronegativity difference between I and Cl), leading to stronger permanent dipole-dipole interactions. Br₂ is nonpolar and only has weaker London dispersion forces, even though both have similar molecular masses. Thus, the polar nature of ICl (Option B) is the correct explanation.
Question
The diagram shows part of the lattice structures of solids X and Y.
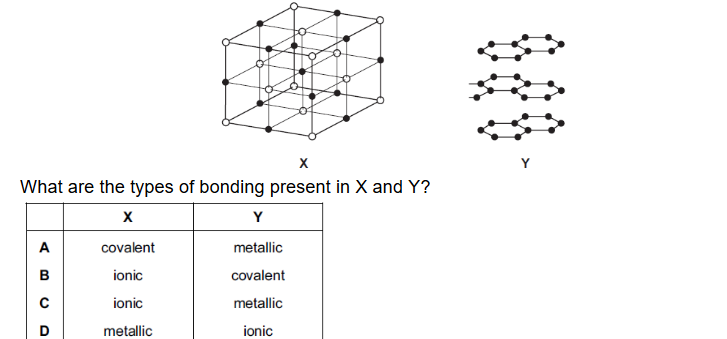
▶️Answer/Explanation
Ans: B
Question
Silver and iodine are both shiny crystalline solids.
Which forces exist between neighboring iodine molecules in solid iodine and particles in solid silver?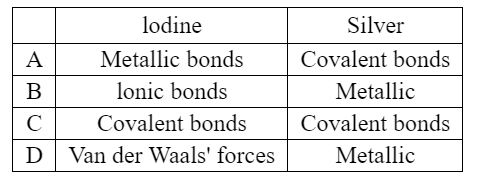
▶️Answer/Explanation
Ans:D
Question
For which substance is the type of bonding not correct?
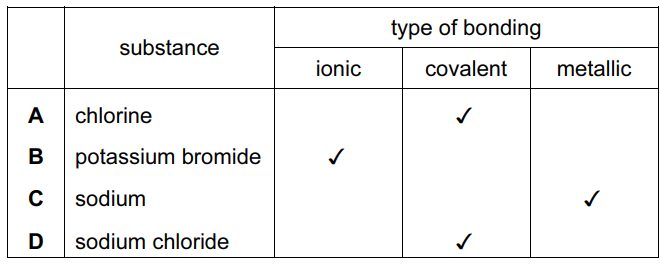
▶️Answer/Explanation
Ans:
C
