(a) State the relative basicities of phenylamine, C₆H₅NH₂, benzylamine, C₆H₅CH₂NH₂, and ammonia, NH₃, in aqueous solution. Explain your answer.

(b) An excess of \(Br_2(aq)\) is added to separate samples of \(C_6H_5NH_2\) and benzene, \(C_6H_6\).
(i) \(C_6H_5NH_2\) reacts readily with \(Br_2 (aq)\) to form organic product M. State the expected observations for this reaction. Draw the structure of M.
(ii) \(C_6H_6\) does not react with \(Br_2 (aq)\). Suggest why \(Br_2 (aq)\) reacts with \(C_6H_5NH_2\) but not with \(C_6H_6\).
(c) Explain why benzamide, C₆H₅CONH₂, is a much weaker base than ammonia, NH₃.
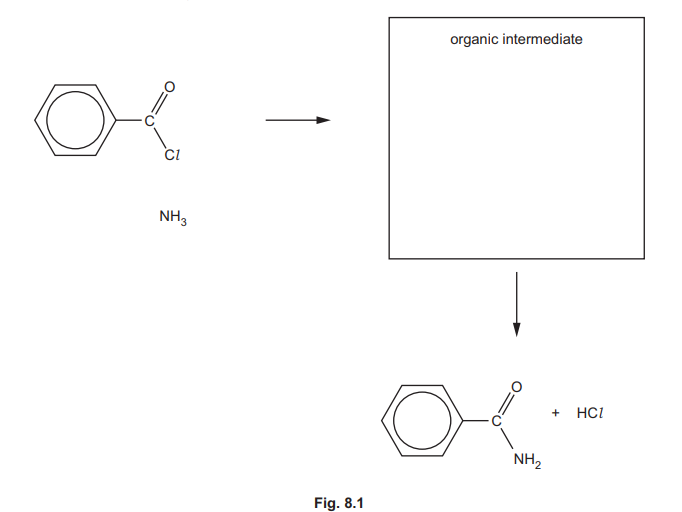
(d) C₆H₅CONH₂ is formed by reacting benzoyl chloride, \(C_6H_5COCl\), with \(NH_3\). Complete the mechanism in Fig. 8.1 for the reaction of \(C_6H_5COCl\) with \(NH_3\). Include all relevant lone pairs of electrons, curly arrows, charges and dipoles. Draw the structure of the organic intermediate.
(e) Phenylalanine, C₆H₅CH₂CH(NH₂)COOH, is an amino acid with an isoelectric point of 5.5.
(i) State what is meant by isoelectric point.
(ii) Draw the structure of C₆H₅CH₂CH(NH₂)COOH at pH 10.
(f) C₆H₅CH₂CH(NH₂)COOH and alanine, \(CH_3CH(NH_2)COOH\), react to form a dipeptide containing both amino acid residues. Draw the structure of this dipeptide. The peptide functional group formed should be displayed.
▶️ Answer/Explanation
(a) Benzylamine > Ammonia > Phenylamine
Explanation:
- Benzylamine’s alkyl group (\(CH_2\)) donates electrons via the +I effect, increasing basicity.
- Ammonia has no electron-withdrawing or donating groups.
- Phenylamine’s lone pair delocalizes into the benzene ring, reducing availability for protonation.
(b)(i) Observations: White precipitate (2,4,6-tribromoaniline) forms; brown bromine color decolorizes.
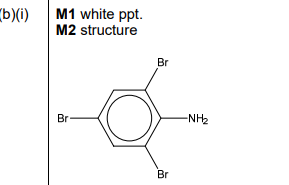
Explanation: The \(-NH_2\) group activates the benzene ring, enabling electrophilic substitution at the 2, 4, and 6 positions.
(b)(ii) \(C_6H_5NH_2\) reacts because the \(-NH_2\) group donates electron density to the ring, polarizing \(Br_2\). Benzene lacks an activating group and requires a catalyst (e.g., FeBr₃) for bromination.
Explanation: The lone pair on \(-NH_2\) increases the ring’s electron density, facilitating electrophilic attack.
(c) Benzamide’s lone pair on nitrogen is delocalized into the carbonyl (\(C=O\)) group, reducing its availability to accept protons.
Explanation: The adjacent carbonyl withdraws electron density via resonance, making the nitrogen less basic than in NH₃.
(d) Nucleophilic acyl substitution mechanism:
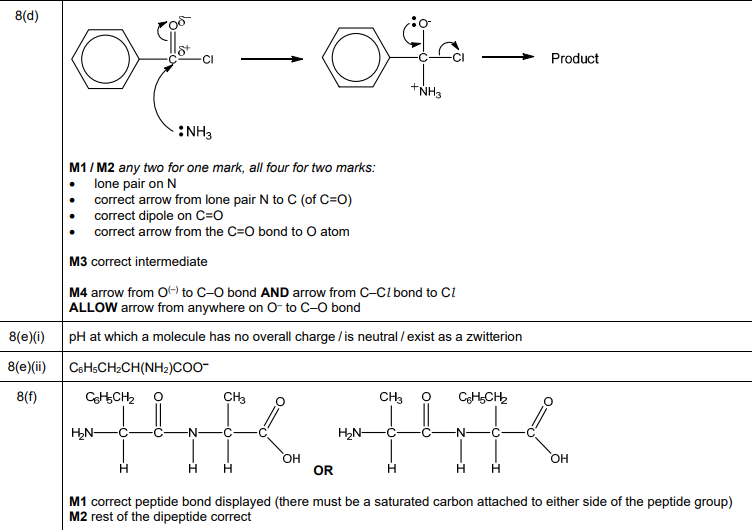
Explanation: NH₃ attacks the electrophilic carbonyl carbon, forming a tetrahedral intermediate. Chloride leaves, yielding benzamide.
(e)(i) Isoelectric point (pI): The pH at which an amino acid has no net charge (zwitterion form).
Explanation: At pI (5.5 for phenylalanine), the \(-NH_3^+\) and \(-COO^-\) groups are balanced.
(e)(ii) At pH 10, phenylalanine is deprotonated: \(C_6H_5CH_2CH(NH_2)COO^-\).
Explanation: The \(-COOH\) group loses \(H^+\) (pKa ~2.2), and \(-NH_3^+\) deprotonates to \(-NH_2\) (pKa ~9.1).
(f) Dipeptide structure (phenylalanine-alanine or alanine-phenylalanine):

Explanation: Condensation reaction forms a peptide bond (\(-CONH-\)) between the carboxyl and amino groups of the two amino acids.
