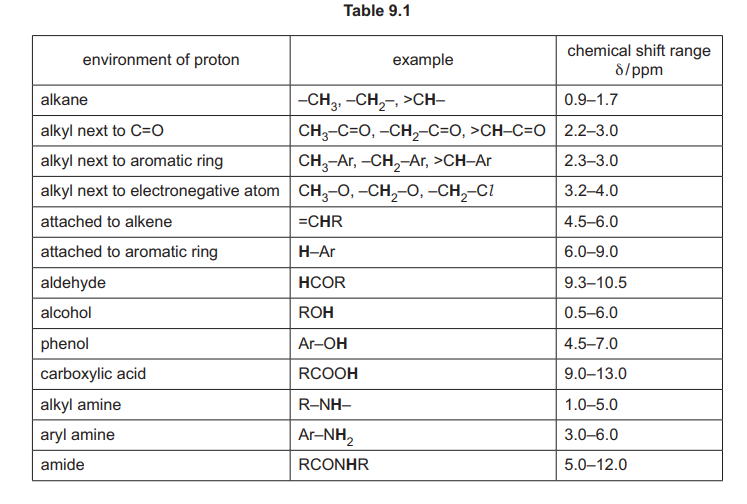
(a) Explain why trichloroethanoic acid, CCl₃COOH, is more acidic than ethanoic acid, CH₃COOH.
(b) Acyl chlorides are formed by reacting carboxylic acids with thionyl chloride, SOCl₂.
(i) Ethanedioyl chloride, (COCl)₂, can be prepared by reacting ethanedioic acid, (COOH)₂, with an excess of SOCl₂. Write an equation for this reaction.
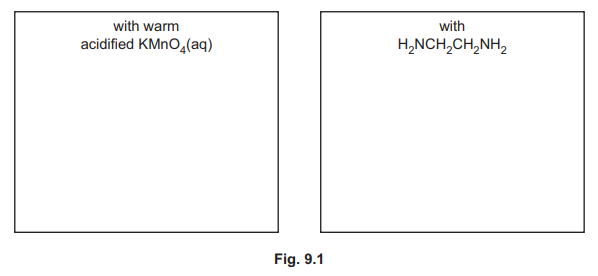
(ii) Samples of (COCl)₂ are reacted separately with an excess of warm acidified KMnO₄ (aq) and with H₂NCH₂CH₂NH₂. The carbon‑containing product from the reaction with H₂NCH₂CH₂NH₂ has the molecular formula \(C_4H_6N_2O_2\). Complete the boxes in Fig. 9.1 to suggest the structure of the carbon‑containing product in each reaction.
(iii) A polyester can be synthesised from the reaction of (COCl)₂ with ethane‑1,2‑diol, HOCH₂CH₂OH. Draw two repeat units of the polymer formed. Any functional groups should be displayed.
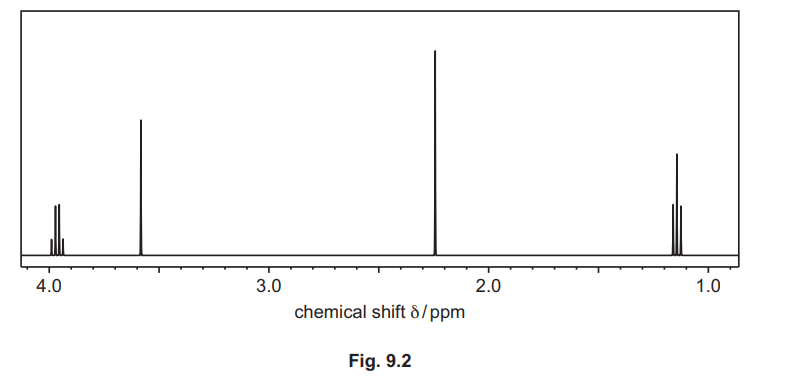
(c) Compound H, C₆H₁₀O₃, reacts with alkaline I₂ (aq) to form yellow precipitate J but does not react with Na₂CO₃ (aq). The proton (1H) NMR spectrum of H in CDCl₃ is shown in Fig. 9.2.
(i) Identify yellow precipitate J.
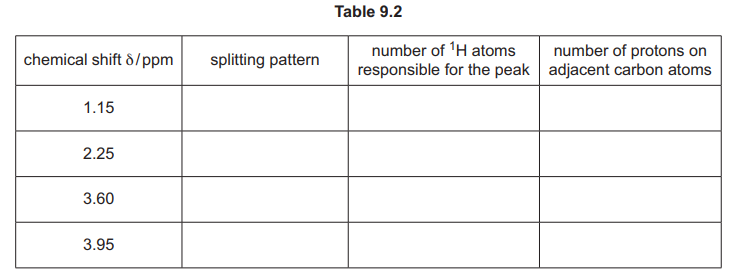
(ii) Complete Table 9.2 for the proton \((^1H)\) NMR spectrum of H, C₆H₁₀O₃.
(iii) Suggest a structure for H, \(C_6H_{10}O_3\).
▶️ Answer/Explanation
(a) Due to the electron-withdrawing effect (–I effect) of chlorine atoms, which stabilizes the carboxylate anion (CCl₃COO⁻) and weakens the O–H bond.
Explanation: The three chlorine atoms in trichloroethanoic acid withdraw electron density via induction, making the conjugate base more stable and the acid more acidic compared to ethanoic acid.
(b)(i) \((COOH)_2 + 2SOCl_2 \to (COCl)_2 + 2HCl + 2SO_2\)
Explanation: Thionyl chloride (SOCl₂) converts both carboxylic acid groups into acyl chlorides, releasing HCl and SO₂ as byproducts.
(b)(ii)
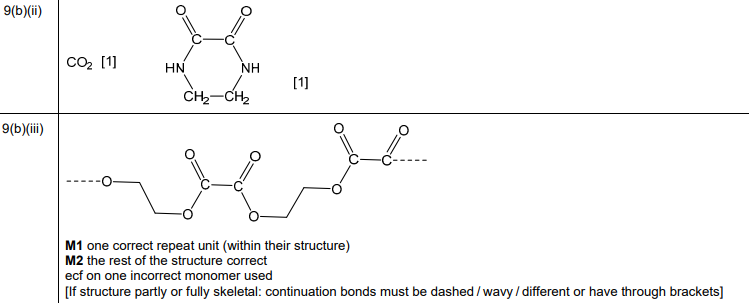
Explanation:
- With KMnO₄: Oxidative cleavage yields CO₂ (carbon-containing product).
- With H₂NCH₂CH₂NH₂: Nucleophilic substitution forms a diamide (\(C_4H_6N_2O_2\)).
(b)(iii) Two repeat units of the polyester:

Explanation: The reaction between (COCl)₂ and ethane-1,2-diol forms a polyester with repeating units of \(-CO-CO-O-CH_2-CH_2-O-\).
(c)(i) \(CHI_3\) (triiodomethane / iodoform)
Explanation: The yellow precipitate indicates a positive iodoform test, confirming the presence of a methyl ketone (\(CH_3CO-\)) group.
(c)(ii)
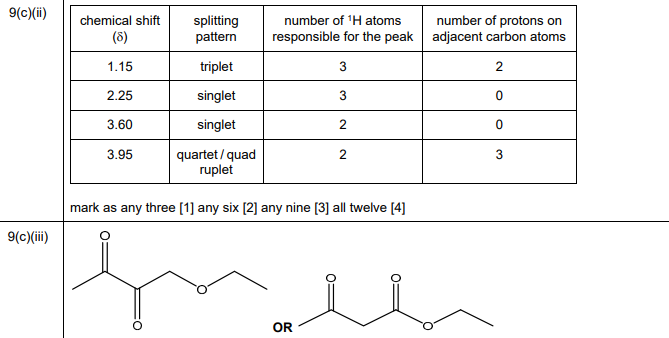
Explanation: The NMR data suggests:
- Singlet at 2.1 ppm (3H): Methyl group adjacent to carbonyl.
- Multiplet at 2.5 ppm (2H): Methylene near carbonyl.
- Triplet at 3.7 ppm (2H): Methylene next to oxygen.
- Singlet at 12.2 ppm (1H): Carboxylic acid proton.
(c)(iii) Structure of H: \(CH_3COCH_2CH_2COOH\) (4-oxopentanoic acid)
Explanation: The NMR spectrum and reactions confirm a methyl ketone (\(CH_3CO-\)) and a carboxylic acid (\(-COOH\)) separated by a \(CH_2\) group.
