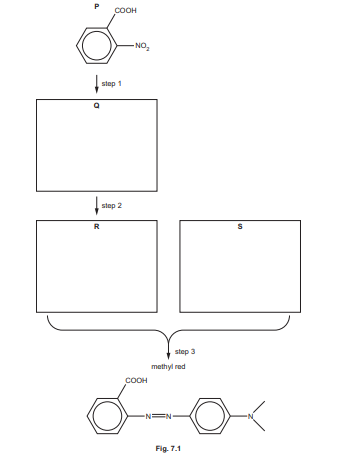
Methyl red can be synthesised as shown in Fig. 7.1.
(a) (i) Give the systematic name of P.
(ii) P can be synthesised as shown in Fig. 7.2.
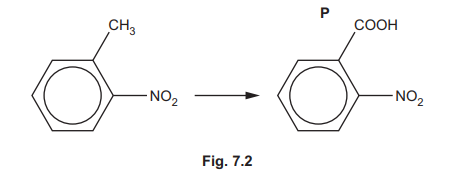
Suggest reagents and conditions for this reaction.
(iii) A student attempts to synthesise P by an alternative route, as shown in Fig. 7.3. Compound T is the major product in this reaction rather than P. 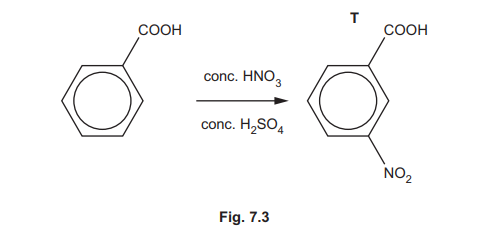
Explain why T is the major product in this reaction.
(b) S reacts in a similar way to phenol in step 3.
(i) Draw the structures of Q, R and S in the boxes in Fig. 7.1.
(ii) Suggest reagents and conditions for steps 1 and 2 in Fig. 7.1.
▶️ Answer/Explanation
(a)(i)
Answer: 2-nitrobenzoic acid OR 2-nitrobenzenecarboxylic acid.
Explanation: The systematic name of P is derived from the parent structure benzoic acid with a nitro group at the 2-position (ortho to the carboxyl group).
(a)(ii)
Answer: Hot/reflux conditions with acidified/alkaline \( KMnO_4 \) (potassium permanganate).
Explanation: The oxidation of a methyl group (\( -CH_3 \)) to a carboxyl group (\( -COOH \)) requires a strong oxidising agent like \( KMnO_4 \) under heating or reflux.
(a)(iii)
Answer: The carboxyl group is electron-withdrawing and meta-directing, favouring nitration at the 3- and 5-positions (T) over the 2-position (P).
Explanation: Electron-withdrawing groups like \( -COOH \) deactivate the benzene ring and direct electrophilic substitution (nitration) to the meta positions, making T the major product.
(b)(i)
Answer: 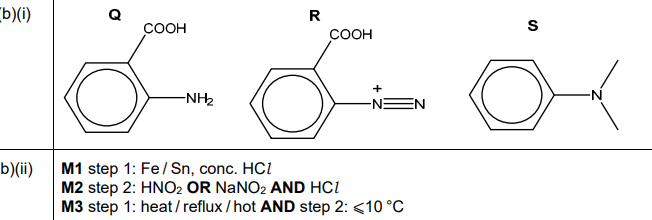
Explanation: Q is the diazonium salt formed from P, R is the intermediate after coupling, and S is the final methyl red structure (as shown in the image).
(b)(ii)
Answer: Step 1: \( NaNO_2 \) and dilute HCl at 0–5 °C (diazotisation).
Step 2: \( N,N \)-dimethylaniline in acidic/alkaline medium (coupling reaction).
Explanation: Step 1 converts the amine to a diazonium salt, while Step 2 involves an azo coupling reaction with \( N,N \)-dimethylaniline to form methyl red.
