Benzene, \(C_6H_6\), reacts with chloroethane, \(C_2H_5Cl\), in the presence of a suitable catalyst to form ethylbenzene, \(C_6H_5C_2H_5\). In the presence of the catalyst, the ion \(C_2H_5^+\) is formed. This ion reacts with benzene.
(a) Complete the equation for the reaction of \(C_2H_5Cl\), with this catalyst to form \(C_2H_5^+\) as one product.
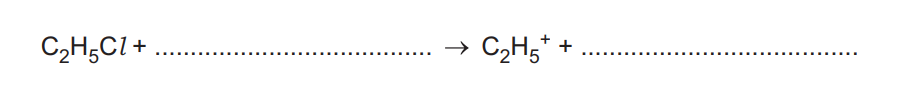
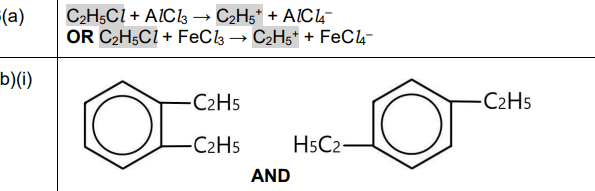
(b) Ethylbenzene reacts with more \(C_2H_5Cl\), forming a mixture containing 1,2-diethylbenzene and 1,4-diethylbenzene.
(i) Draw the structures of 1,2-diethylbenzene and 1,4-diethylbenzene. 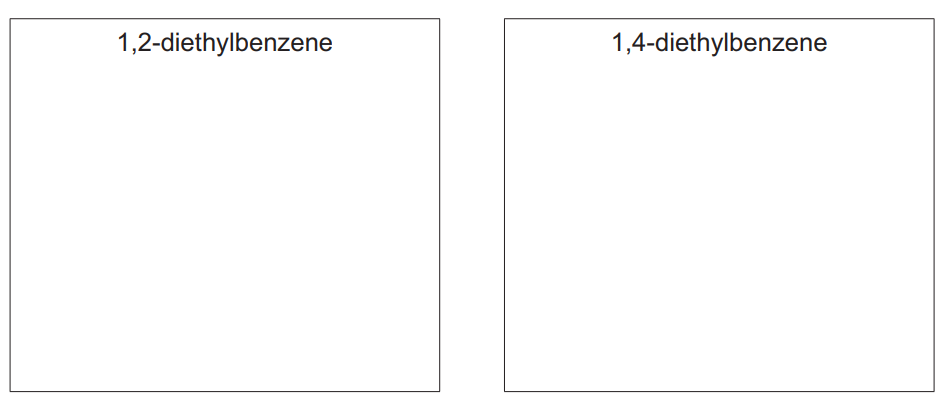
(ii) Explain why there is very little 1,3-diethylbenzene in the product mixture.
(c) 1,2-diethylbenzene can be oxidised to benzene-1,2-dioic acid, \(C_6H_4(COOH)_2\).
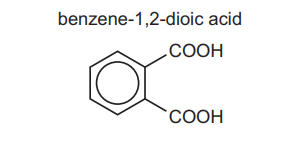
(i) State the reagent and conditions used for this reaction.
(ii) Complete the overall equation for this reaction. An atom of oxygen from the oxidising agent is represented as [O]. All of the atoms in the two ethyl groups are fully oxidised in this reaction 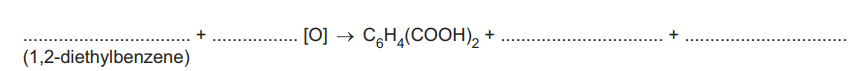
(iii) Predict the number of peaks in the carbon-13 NMR spectrum of benzene-1,2-dioic acid.
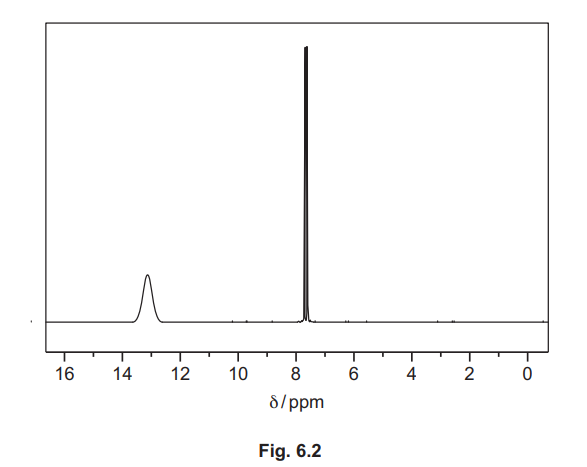
(d) The proton (1H) NMR spectra of ethylbenzene, \(C_6H_5C_2H_5\), in \(CDCl_3\), and of benzene-1,2-dioic acid, \(C_6H_4(COOH)_2\), in \(CDCl_3\) are shown. They have not been identified.
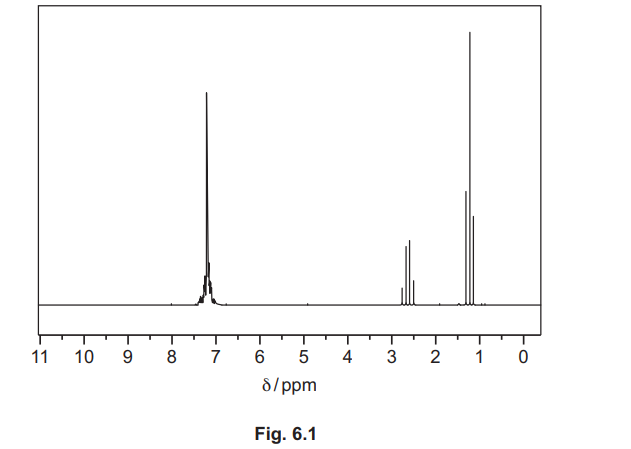
(i) Explain the use of CDCl₃, instead of CHCl₃, as the solvent when obtaining these spectra.
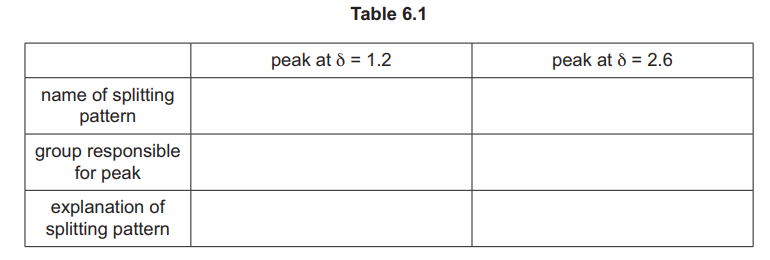
(ii) Identify the substance shown by the spectrum in Fig. 6.1, and complete Table 6.1.
(iii) Identify the substance shown by the spectrum in Fig. 6.2, and complete Table 6.2. 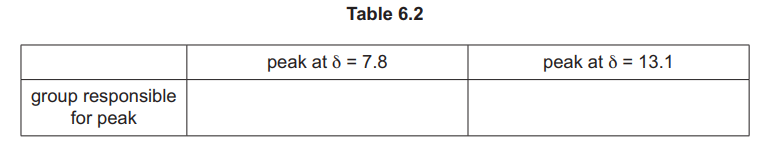
(iv) When \(D_2O\) is used as a solvent, the spectrum obtained is different from the spectrum in Fig. 6.2. Describe this difference and explain your answer.
(e) Benzene-1,2-dioic acid can be used to produce K.
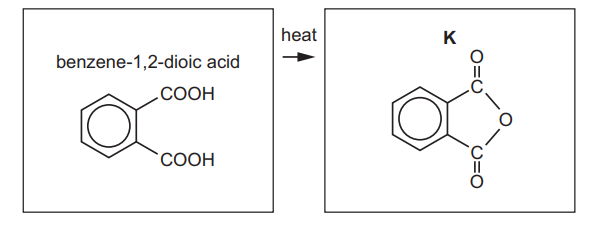
Suggest the name of this type of reaction.
▶️ Answer/Explanation
(a)
Explanation: The reaction involves the formation of the ethyl cation (\(C_2H_5^+\)) from chloroethane (\(C_2H_5Cl\)) in the presence of a catalyst (e.g., AlCl₃). The catalyst facilitates the heterolytic cleavage of the C-Cl bond, releasing \(Cl^-\) and forming \(C_2H_5^+\).
(b)(i)

Explanation: 1,2-diethylbenzene has ethyl groups on adjacent carbons, while 1,4-diethylbenzene has them on opposite sides of the benzene ring. The structures are drawn accordingly.
(b)(ii) Alkyl/ethyl group is 2,4-directing OR ethyl group is electron-donating (positive inductive effect).
Explanation: The ethyl group directs incoming electrophiles to the ortho (2) and para (4) positions due to its electron-donating nature, making 1,3-diethylbenzene a minor product.
(c)(i) Hot alkaline KMnO₄ / MnO₄⁻.
Explanation: The strong oxidizing agent (alkaline KMnO₄) under heating converts the ethyl groups into carboxyl groups (-COOH).
(c)(ii)
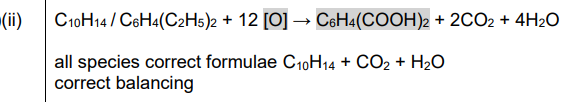
Explanation: The two ethyl groups are fully oxidized to carboxyl groups, consuming 10 [O] atoms in the process.
(c)(iii) 4 peaks.
Explanation: The carbon-13 NMR spectrum shows peaks for the four distinct carbon environments: two carboxyl carbons and two pairs of equivalent aromatic carbons.
(d)(i) CDCl₃ does not cause a peak OR does not interfere with the spectrum.
Explanation: CDCl₃ is used because deuterium (D) does not produce a signal in proton NMR, unlike CHCl₃, which would interfere.
(d)(ii) Ethylbenzene / \(C_6H_5C_2H_5\).
Explanation: The spectrum shows a triplet (CH₃) and quartet (CH₂) due to spin-spin coupling, along with aromatic protons.
(d)(iii) Benzene-1,2-dioic acid / \(C_6H_4(COOH)_2\).
Explanation: The spectrum includes a downfield peak for the COOH proton (~13 ppm) and aromatic protons (~7-8 ppm).
(d)(iv) COOH peak disappears OR peak at 13.1 ppm is removed.
Explanation: In \(D_2O\), the COOH proton exchanges with deuterium, which does not produce a signal in proton NMR.
(e) Dehydration / elimination / (auto)condensation.
Explanation: The reaction involves the loss of water molecules to form a cyclic anhydride, characteristic of a dehydration or condensation reaction.
