(a) (i) State what is meant by partition coefficient, \(K_{pc}\).
(ii) The partition coefficient, \(K_{pc}\), for a compound, X, between carbon disulfide, CS₂, and water is 10.5. 1.85 g of X is dissolved in water and made up to 100.0 cm³ in a volumetric flask. 40.0 cm³ of this aqueous solution is shaken with 25.0 cm³ of CS₂. The mixture is left to reach equilibrium. Calculate the mass of X, in g, extracted into the \(CS_2\) layer.
(b) The compound \(C_6H_6\) has many structural isomers. Four suggested structures of \(C_6H_6\) are shown in Fig. 6.1.
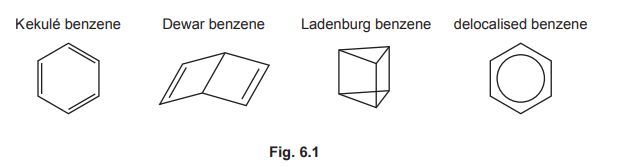
Using Fig. 6.1, complete Table 6.1 to predict the number of carbon atoms that have sp, \(sp^2\), and \(sp^3\) hybridisation in Kekulé benzene, Dewar benzene, and Ladenburg benzene.

(c) Describe the shape of delocalised benzene. Include the geometry of each carbon, the C-C-H bond angle, and the type of bond(s) between the carbon atoms and between the carbon and hydrogen atoms.
(d) Suggest why Dewar benzene and Ladenburg benzene are unstable isomers of \(C_6H_6\).
(e) Complete Table 6.2 to predict the number of peaks in the proton \((^1H)\) NMR spectrum for Dewar benzene, Ladenburg benzene and delocalised benzene.
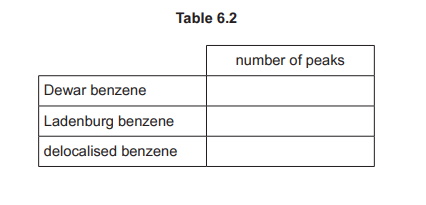
(f) The reaction of phenylethanone with 1,4-dibromobutane, BrCH₂CH₂CH₂CH₂Br, in the presence of FeBr₃ is shown in Fig. 6.2.
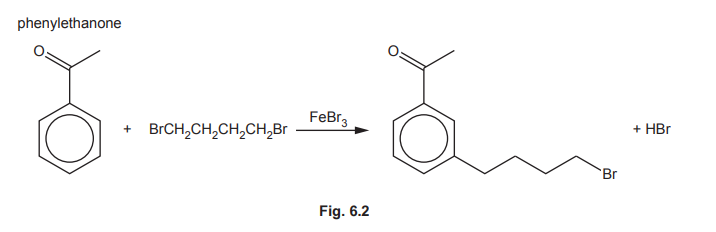
The mechanism of this reaction is similar to that of the alkylation of benzene.
(i) Construct an equation for the formation of the electrophile, BrCH₂CH₂CH₂CH₂⁺.
(ii) Complete the mechanism in Fig. 6.3 for the reaction of phenylethanone with BrCH₂CH₂CH₂CH₂⁺ ions. Include all relevant curly arrows and charges. Draw the structure of the organic intermediate.
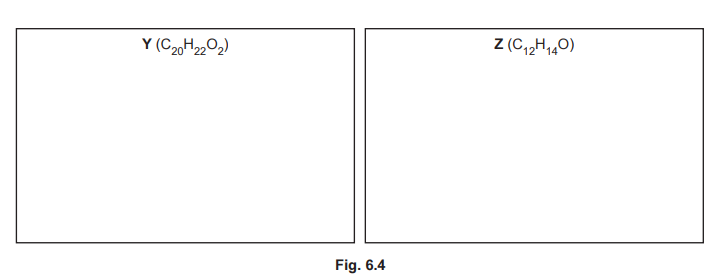
(iii) The reaction shown in Fig. 6.2 forms small amounts of two by-products, Y \((C_{20}H_{22}O_2)\) and Z \((C_{12}H_{14}O)\). Suggest structures for Y and Z in the boxes in Fig. 6.4.
▶️ Answer/Explanation
(a)(i) The partition coefficient, \(K_{pc}\), is the ratio of the concentrations of a solute in two immiscible solvents at equilibrium.
Explanation: It describes how a solute distributes itself between two phases, such as organic and aqueous layers.
(a)(ii) 0.642 g
Explanation: Using the partition coefficient formula \(K_{pc} = \frac{[X]_{CS_2}}{[X]_{H_2O}}\), we set up the equation \(10.5 = \frac{(y/25)}{(0.74 – y)/40}\), where \(y\) is the mass extracted into CS₂. Solving gives \(y = 0.642 \, \text{g}\).
(b)
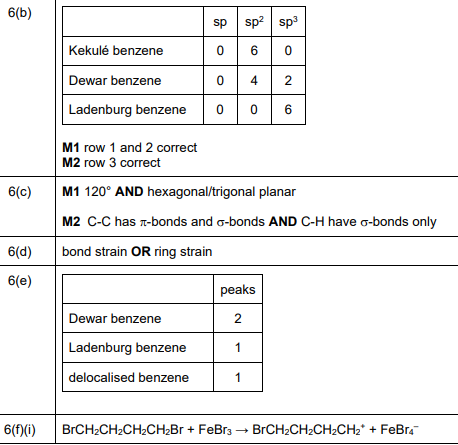
Explanation: Kekulé benzene has 6 \(sp^2\) carbons. Dewar benzene has 4 \(sp^2\) and 2 \(sp^3\) carbons. Ladenburg benzene has 2 \(sp\), 2 \(sp^2\), and 2 \(sp^3\) carbons.
(c) Delocalised benzene is planar with each carbon having trigonal planar geometry. The C-C-H bond angle is 120°. The C-C bonds are delocalised \(\pi\)-bonds, and C-H bonds are sigma (\(\sigma\)) bonds.
Explanation: The ring is flat due to \(sp^2\) hybridisation, with equal bond lengths from electron delocalisation.
(d) Dewar benzene and Ladenburg benzene are unstable due to ring strain and lack of aromaticity (delocalisation).
Explanation: Their structures introduce angle strain and prevent the stability conferred by aromatic \(\pi\)-electron delocalisation in benzene.
(e)
Explanation: Dewar benzene has 2 peaks (2 types of H), Ladenburg benzene has 3 peaks (3 types of H), and delocalised benzene has 1 peak (all H equivalent).
(f)(i) \(\text{BrCH}_2\text{CH}_2\text{CH}_2\text{CH}_2\text{Br} + \text{FeBr}_3 \rightarrow \text{BrCH}_2\text{CH}_2\text{CH}_2\text{CH}_2^+ + \text{FeBr}_4^-\)
Explanation: FeBr₃ acts as a Lewis acid, polarising the C-Br bond to generate the electrophile.
(f)(ii)
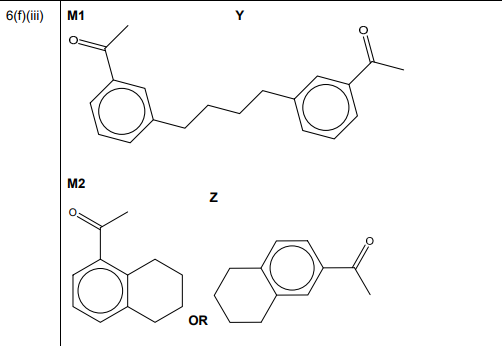
Explanation: The mechanism involves electrophilic attack on the benzene ring, forming a carbocation intermediate, followed by deprotonation to restore aromaticity.
(f)(iii) Y is a dimer (two phenylethanone units bridged by the dibromobutane chain). Z is a monosubstituted product (one phenylethanone with a butyl group).
Explanation: Y forms from two electrophilic attacks, while Z results from a single substitution.
