The amino acid serine, \(HOCH_2CH(NH_2)COOH\), exists in two optically active forms. These optical isomers, isomer P and isomer Q, are shown in Fig. 8.1.
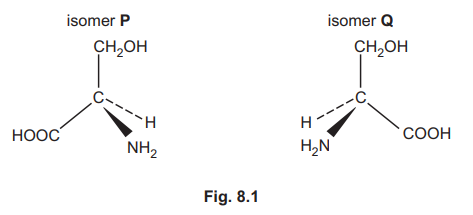
(a) Isomer P and isomer Q have identical physical and chemical properties, with the exception of two specific properties. One of these two properties is their differing effect on plane polarised light. State the other property by which they differ.
(b) A solution of pure isomer P of a particular concentration rotates plane polarised light by 5.0° in a clockwise direction. Describe how a solution of pure isomer Q of the same concentration affects plane polarised light.
(c) State another term, in addition to stereoisomers, optical isomers and non-superimposable mirror images, which can be used to describe this pair of chiral compounds, isomer P and isomer Q.
(d) Give the term used to describe a mixture containing equal amounts of isomer P and isomer Q.
(e) Describe one way in which a single pure optical isomer of serine can be produced, instead of making a mixture of isomer P and isomer Q.
(f) Complete Table 8.1 to describe the peaks seen in the proton \((^1H)\) NMR spectrum of HOCH₂CH(NH₂)COOH dissolved in D₂O. Use as many rows in Table 8.1 as you need to, leaving the other rows blank.
(g) Proline is a naturally occurring amino acid. The skeletal formula of proline is shown.
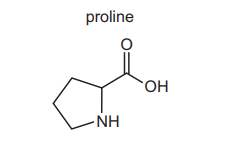
State the number of peaks in the carbon-13 \((^{13}C)\) NMR spectrum of proline.
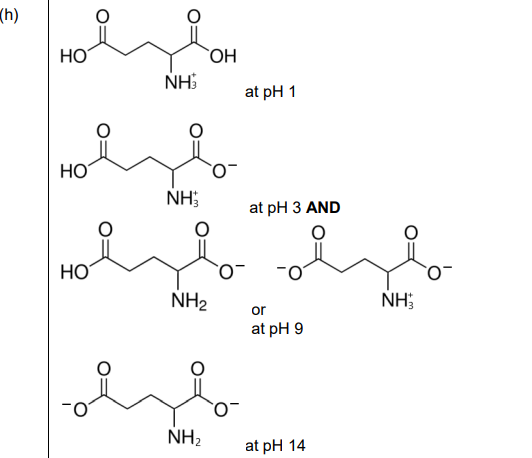
(h) Glutamic acid is a naturally occurring amino acid. The skeletal formula of glutamic acid is shown.
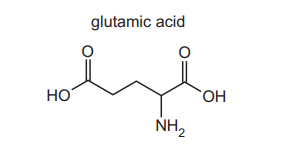
The isoelectric point of glutamic acid is pH 3. A sample of glutamic acid is dissolved in a solution of pH 1. A strong alkali is then added until the pH of the mixture reaches pH 14. During this process all possible ionised forms of glutamic acid are present at different times, depending on the pH of the solution. Complete the boxes below to show four different ionised forms of glutamic acid that are present at the stated pH values.
▶️ Answer/Explanation
(a) Biological activity.
Explanation: Enantiomers (P and Q) may interact differently with biological systems (e.g., enzymes or receptors) due to their chiral nature, despite having identical physical and chemical properties otherwise.
(b) Rotates plane polarised light 5.0° anticlockwise.
Explanation: Enantiomers rotate plane-polarized light equally but in opposite directions. If P rotates +5.0° (clockwise), Q must rotate −5.0° (anticlockwise).
(c) Enantiomers.
Explanation: This term specifically describes non-superimposable mirror-image isomers that rotate plane-polarized light in opposite directions.
(d) Racemic mixture.
Explanation: A 1:1 mixture of enantiomers (P and Q) is called a racemate or racemic mixture, which does not rotate plane-polarized light.
(e) Use a chiral catalyst or enzyme.
Explanation: Chiral catalysts or enzymes selectively produce one enantiomer (e.g., P or Q) by favoring one transition state over its mirror image.
(f)
Explanation: The \(^1H\) NMR spectrum shows:
- CH₂ (2 protons): Doublet (split by 1 proton on adjacent carbon).
- CH (1 proton): Triplet (split by 2 protons on adjacent carbon).
(g) 5 peaks.
Explanation: Proline has 5 unique carbon environments (highlighted in its structure), each giving a distinct peak in the \(^{13}C\) NMR spectrum.
(h)

Explanation: Glutamic acid ionizes as follows:
- pH 1: Fully protonated (COOH groups and NH₃⁺).
- pH 3 (isoelectric point): Zwitterion (NH₃⁺ and COO⁻).
- pH 7–9: One COOH deprotonates.
- pH 14: Fully deprotonated (all COO⁻ and NH₂).
