Four esters, A, B, C and D, with the molecular formula \(C_6H_{12}O_2\) are shown in Fig. 7.1.
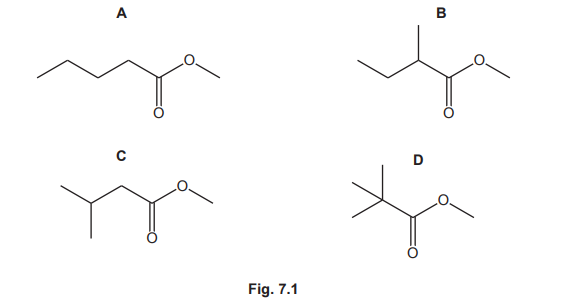
(a) Give the systematic name of ester A.
(b) A mixture of these esters, A, B, C and D, is analysed by gas–liquid chromatography. The chromatogram produced is shown in Fig. 7.2. The number above each peak represents the area under the peak. The area under each peak is proportional to the mass of the respective ester in the mixture.
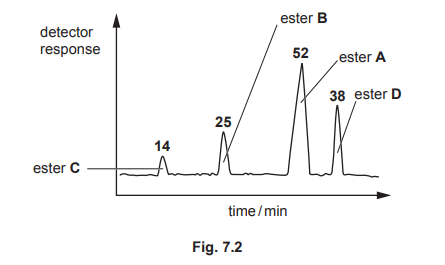
(i) State what is meant by retention time.
(ii) Calculate the percentage by mass of ester D in the original mixture.
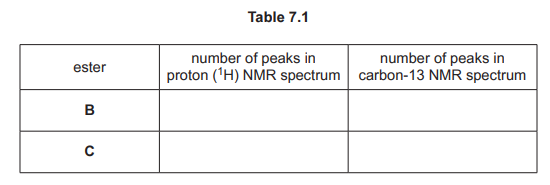
(c) Separate samples of the esters, A, B, C and D, are analysed using proton \((^1H)\) NMR and carbon-13 NMR spectroscopy.
(i) Complete Table 7.1 to show the number of peaks in each NMR spectrum for esters B and C.
(ii) Identify all of the esters from A, B, C and D that have at least one triplet peak in their proton \((^1H)\) NMR spectrum.
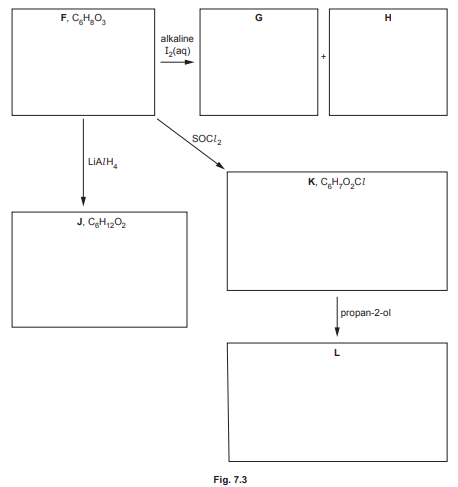
(d) Compound F, \(C_6H_8O_3\), shows stereoisomerism and effervesces with \(Na_2CO_3(aq)\).
Compound F reacts with alkaline \(I_2(aq)\) to form yellow precipitate G and compound H.
Compound F reacts with \(LiAlH_4\) to form compound J, \(C_6H_{12}O_2\).
Compound F reacts with SOCl₂ to form compound K, C₆H₇O₂Cl.
Compound K reacts with propan-2-ol to form compound L.
Draw the structures of compounds F, G, H, J, K and L in the boxes in Fig. 7.3.
▶️ Answer/Explanation
(a) The systematic name of ester A is methyl pentanoate.
Explanation: A is formed from methanol (methyl) and pentanoic acid (pentanoate), giving the IUPAC name methyl pentanoate.
(b)(i) Retention time is the time taken for a compound to travel from the injection point to the detector in gas chromatography.
Explanation: It is characteristic of each compound under specific conditions.
(b)(ii) The percentage by mass of ester D is 29.5%.
Explanation: The area under peak D is 59, and the total area is 200 (45 + 56 + 40 + 59). Thus, %D = (59/200) × 100 = 29.5%.
(c)(i) The completed Table 7.1 is:
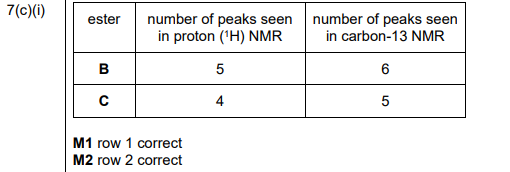
Explanation:
- Ester B: 3 peaks in \(^1H\) NMR (due to 3 proton environments) and 4 peaks in \(^{13}C\) NMR (4 carbon environments).
- Ester C: 2 peaks in \(^1H\) NMR (2 proton environments) and 3 peaks in \(^{13}C\) NMR (3 carbon environments).
(c)(ii) Esters A and B have at least one triplet peak in their \(^1H\) NMR spectrum.
Explanation: Triplets arise from protons adjacent to \(-CH_2-\) groups (e.g., \(-OCH_2CH_3\) in A and B).
(d) The structures of compounds F, G, H, J, K, and L are:
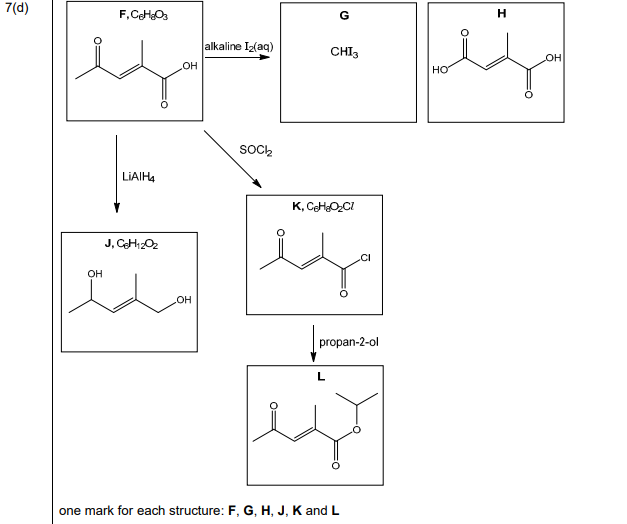
Explanation:
- F: Stereoisomeric compound with \(-COOH\) (effervesces with \(Na_2CO_3\)) and \(-COCH_3\) (reacts with \(I_2\) to form iodoform, G).
- G: Yellow precipitate is iodoform (\(CHI_3\)).
- H: Sodium salt of the acid formed after iodoform reaction.
- J: Reduction product (\(C_6H_{12}O_2\)) from \(LiAlH_4\).
- K: Acyl chloride formed with SOCl₂.
- L: Ester formed from K and propan-2-ol.
