Question
The proton NMR spectrum of compound $\mathbf{E}$ in the solvent $\mathrm{CDC} l_3$ is shown. The molecular formula of compound $\mathbf{E}$ is $\mathrm{C}_9 \mathrm{H}_{10} \mathrm{O}_2$.
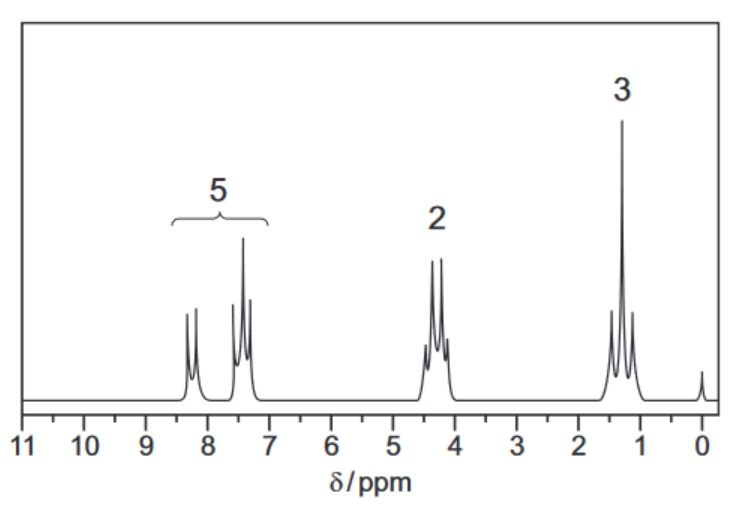
(a) Explain why $\mathrm{CDCl}_3$ is used as a solvent instead of $\mathrm{CHCl}_3$.[1]
(b) Explain why TMS is added to give the small peak at chemical shift $\delta=0$.[1]
(c) Compound $\mathbf{E}$ is hydrolysed by hot $\mathrm{NaOH}(\mathrm{aq})$, giving two organic products only. One of these products is ethanol.
Name the functional group in compound $\mathbf{E}$ that is hydrolysed by hot $\mathrm{NaOH}(\mathrm{aq})$.[1]
(d) (i) Describe and explain the splitting patterns of the peaks at $\delta=1.4$ and $\delta=4.3$.
splitting pattern at $\delta=1.4$
reason for splitting pattern at $\delta=1.4$
splitting pattern at $\delta=4.3$
reason for splitting pattern at $\delta=4.3$[2]
(ii) Each molecule of compound $\mathbf{E}$ contains five protons which give rise to the peaks between $\delta=7.0$ and $\delta=8.5$.
Identify the functional group in compound $\mathbf{E}$ which contains these protons.[1]
(iii) Give the structural formula of compound $\mathbf{E}$.[1]
(e) The mass spectrum of compound $\mathbf{E}$ includes fragment ions with m/e values of 29 and 77 . Give the formulae of these fragment ions.
fragment ion with $m / e=29$
fragment ion with $m / e=77$[5][Total: 9]
▶️Answer/Explanation
Ans:
(a) (because $\mathrm{CDC} l_3 /$ it) does not give a peak [1] OR because $\mathrm{CHCl}_3$ does give a peak
(b) as a standard / reference for (chemical shift measurements) [1]
(c) ester [1] 1
9(d)(i) • (δ = 1.4) triplet
• (δ = 1.4) two H on neighbouring C atom
• (δ = 4.3) quartet / quadruplet
• (δ = 4.3) three H on neighbouring C atom
mark as $\bullet \checkmark \bullet \checkmark[2]$
9(d)(ii) aryl group / arene / phenyl [1]
(d)(iii)
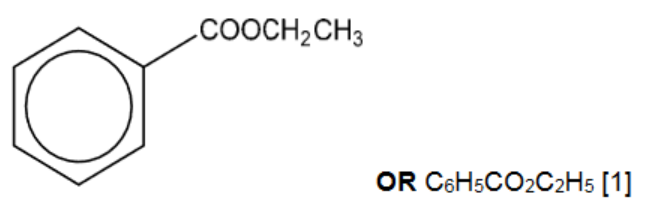
(e)$\mathrm{CH}_3 \mathrm{CH}_2^{+} / \mathrm{C}_2 \mathrm{H}_5^{+}[1]$
$\mathrm{C}_6 \mathrm{H}_5^{+}[1]$
