(a) Define enthalpy change of formation.
(b) Iron is made when iron(III) oxide is heated with carbon monoxide, as shown by reaction 2.
reaction 2 Fe₂O₃ + 3CO → 2 Fe + 3 CO₂
Table 4.1 shows enthalpy change of formation data measured at 298K and 101kPa.
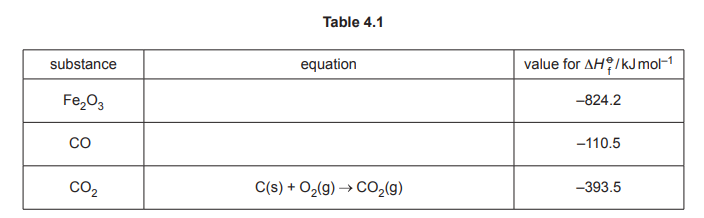
(i) Complete Table 4.1 by adding equations with relevant state symbols to represent:
• standard enthalpy change of formation for Fe₂O₃.
• standard enthalpy change of formation for CO.
(ii) Use the data in Table 4.1 to calculate the enthalpy change of reaction, \(∆H_r\), in \(kJmol^{–1}\), for reaction 2. Show your working
▶️ Answer/Explanation
(a) The enthalpy change of formation is the energy change when one mole of a compound is formed from its elements in their standard states under standard conditions (298K and 101kPa).
(b)(i)
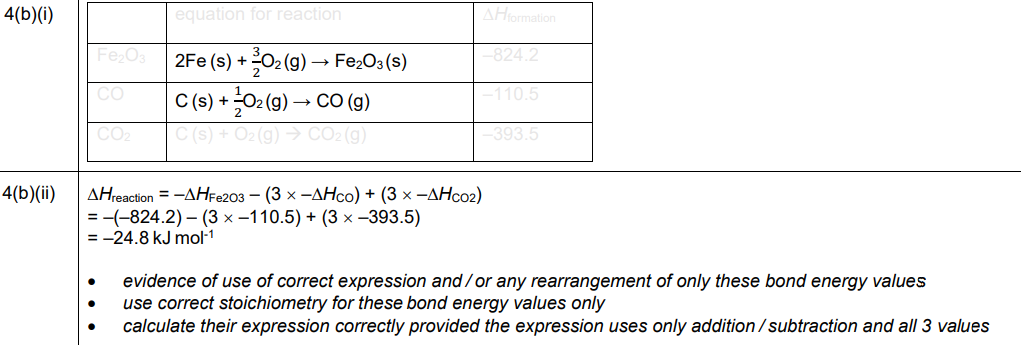
Explanation: The standard enthalpy change of formation for Fe₂O₃ is represented as \(2Fe(s) + \frac{3}{2}O_2(g) \rightarrow Fe_2O_3(s)\), and for CO as \(C(s) + \frac{1}{2}O_2(g) \rightarrow CO(g)\).
(b)(ii) The enthalpy change of reaction, \(∆H_r\), is calculated using the formula:
\[ ∆H_r = \sum ∆H_f(\text{products}) – \sum ∆H_f(\text{reactants}) \]
Substituting the values from Table 4.1:
\[ ∆H_r = [2(0) + 3(-393.5)] – [1(-824.2) + 3(-110.5)] = -1180.5 + 824.2 + 331.5 = -24.8 \, kJmol^{-1} \]
Final Answer: The enthalpy change of reaction is \(\boxed{-24.8 \, kJmol^{-1}}\).
