\(N_2(g)\) reacts with \(H_2(g)\) in the Haber process, as shown in reaction 1.
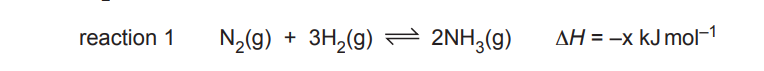
Table 2.1 shows the different conditions used to produce three equilibrium mixtures, A, B and C.
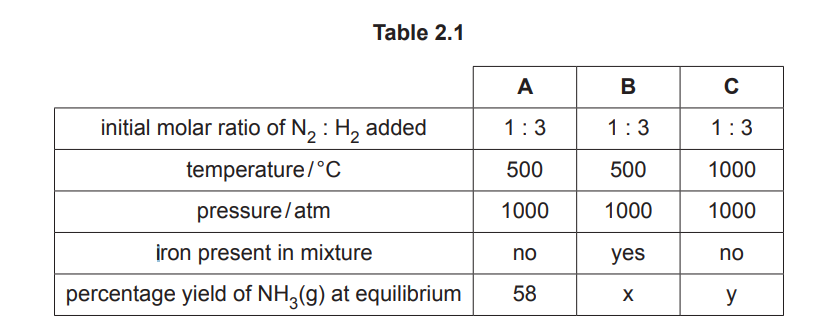
(a) Describe and explain the change, if any, to the percentage yield of NH₃(g) produced in B compared to A.
(b) (i) Describe and explain the change, if any, to the percentage yield of NH₃(g) produced in C compared to A.
(ii) Describe and explain the change to the rate of the forward reaction that occurs to establish the equilibrium in C compared to A. You do not need to refer to the Boltzmann distribution in your answer.
(c) (i) Write an expression for the equilibrium constant, \(K_p\), for reaction 1. State the units.
(ii) Equilibrium mixture D is made when 1.0mol of \(N_2(g)\) and 3.0mol of \(H_2(g)\) are added to a sealed container at 750°C and 1000 atm and left to reach equilibrium. This mixture contains 1.16mol of NH₃(g). Calculate the mole fraction of NH₃(g) in D.
(iii) The mole fraction of \(N_2(g)\) is 0.625 in a new equilibrium mixture, E. Calculate the partial pressure of \(N_2(g)\) in E when the total pressure is 1000 atm.
(d) When oxides of nitrogen escape into the atmosphere they may be involved in:
• formation of acid rain from sulfur dioxide
• formation of photochemical smog.
(i) Identify the role of NO and \(NO_2\) in the formation of \(H_2SO_4\) from \(SO_2\) in the atmosphere to produce acid rain. Use relevant equations to support your answer.
(ii) Outline how NO and \(NO_2\) may contribute to the formation of photochemical smog.
▶️ Answer/Explanation
(a) The percentage yield of NH₃ remains unchanged (58%) in B compared to A.
Explanation: A catalyst (iron in this case) does not affect the position of equilibrium or the yield; it only speeds up the attainment of equilibrium.
(b)(i) The percentage yield of NH₃ decreases in C compared to A.
Explanation: The forward reaction is exothermic (\(\Delta H = -92 \text{ kJ/mol}\)). Increasing the temperature (from 450°C to 550°C) shifts the equilibrium to the left (Le Chatelier’s principle), reducing NH₃ yield.
(b)(ii) The rate of the forward reaction increases in C compared to A.
Explanation: Higher temperature provides more molecules with energy greater than the activation energy (\(E > E_a\)), increasing the frequency of effective collisions and thus the reaction rate.
(c)(i) \(K_p = \frac{(p_{\text{NH}_3})^2}{(p_{\text{N}_2})(p_{\text{H}_2})^3}\), Units: \(\text{atm}^{-2}\)
Explanation: The equilibrium constant \(K_p\) is expressed in terms of partial pressures. The units are derived from the stoichiometric coefficients.
(c)(ii) Mole fraction of NH₃ = 0.41
Explanation: At equilibrium, moles of \(N_2 = 0.42\), \(H_2 = 1.26\), and \(NH_3 = 1.16\). Total moles = \(0.42 + 1.26 + 1.16 = 2.84\). Mole fraction of NH₃ = \(\frac{1.16}{2.84} = 0.41\).
(c)(iii) Partial pressure of \(N_2\) = 625 atm
Explanation: Partial pressure = Mole fraction × Total pressure = \(0.625 \times 1000 \text{ atm} = 625 \text{ atm}\).
(d)(i) NO and NO₂ act as catalysts in the oxidation of SO₂ to H₂SO₄.
Explanation: The catalytic cycle involves:
\(\text{SO}_2 + \text{NO}_2 \rightarrow \text{NO} + \text{SO}_3\)
\(\text{SO}_3 + \text{H}_2\text{O} \rightarrow \text{H}_2\text{SO}_4\)
\(\text{2NO} + \text{O}_2 \rightarrow \text{2NO}_2\) (regenerates NO₂).
(d)(ii) NO and NO₂ contribute to photochemical smog by reacting with VOCs.
Explanation: In sunlight, NO₂ photodissociates to form NO and O atoms, which react with hydrocarbons to form ozone and PAN (peroxyacyl nitrates), key components of smog.
