In alkaline solution, MnO₄⁻ ions oxidise SO₃²⁻ ions to SO₄²⁻ ions. The MnO₄⁻ ions are reduced to MnO₂. What is the ratio of the two ions in the balanced chemical equation for this reaction?

▶️ Answer/Explanation
Ans: A
To balance the redox reaction, we first write the half-reactions:
Oxidation: \(\text{SO}_3^{2-} \rightarrow \text{SO}_4^{2-} + 2e^-\)
Reduction: \(\text{MnO}_4^- + 2\text{H}_2\text{O} + 3e^- \rightarrow \text{MnO}_2 + 4\text{OH}^-\)
Balancing the electrons, we multiply the oxidation half-reaction by 3 and the reduction half-reaction by 2. The ratio of \(\text{MnO}_4^-\) to \(\text{SO}_3^{2-}\) is 2:3, which corresponds to option A.
The equation for the reaction of aqueous thiosulfate ions, \(S_2O_3^{2–}\), and aqueous dioxo-vanadium ions, \(VO_2^+\), is shown.
\(2S_2O_3^{2–} + xVO_2^+ + yH^+ \to S_4O_6^{2–} + zVO_2^+ + 2H_2O\)
Which row shows two correct statements about the equation for this reaction?
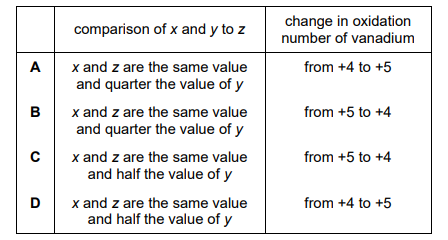
▶️ Answer/Explanation
Ans: C
To balance the given redox reaction:
- Oxidation half-reaction (thiosulfate to tetrathionate):
\(2S_2O_3^{2–} \to S_4O_6^{2–} + 2e^–\) - Reduction half-reaction (vanadium reduction):
\(VO_2^+ + 2H^+ + e^– \to VO^{2+} + H_2O\)
Balancing electrons and combining the half-reactions gives:
\(2S_2O_3^{2–} + 4VO_2^+ + 4H^+ \to S_4O_6^{2–} + 4VO^{2+} + 2H_2O\)
Thus, \(x = 4\), \(y = 4\), and \(z = 4\). The correct row is C, where \(x = 4\) and \(z = 4\).
Lithium reacts with nitrogen at room temperature to form solid Li₃N. Three vessels of equal volume are connected by taps 1 and 2 as shown.
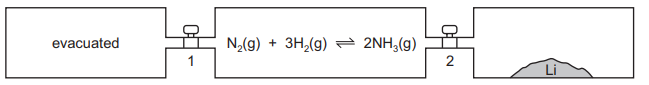
At the start, taps 1 and 2 are closed, the left-hand vessel is evacuated, the middle vessel has the indicated reaction at equilibrium and the right-hand vessel contains lithium only. Which action would allow the equilibrium mixture to contain the most ammonia?
▶️ Answer/Explanation
Ans: A
The equilibrium mixture in the middle vessel is given as: \[ 6Li(s) + N_2(g) \rightleftharpoons 2Li_3N(s) \] To maximize ammonia (NH₃), we must consider Le Chatelier’s Principle. Since the question mentions ammonia, we assume a side reaction where Li₃N reacts with water vapor (if present) to produce NH₃. However, the system is closed, and opening taps would introduce disturbances:
- Option A (Keep taps closed): The equilibrium remains undisturbed, and no additional Li (from the right vessel) or vacuum (from the left vessel) affects the system.
- Other options (B, C, D): Introducing Li (via tap 2) consumes N₂, shifting equilibrium left and reducing NH₃. Opening tap 1 (vacuum) reduces N₂ pressure, also shifting equilibrium left.
Thus, keeping both taps closed (Option A) preserves the equilibrium and maximizes NH₃.
When 0.20 mol of hydrogen gas and 0.15 mol of iodine gas are heated at 723K until equilibrium is established, the equilibrium mixture contains 0.26 mol of hydrogen iodide. The equation for the reaction is as follows.
\(H_2(g) + I_2(g) \to 2HI(g)\)
What is the correct expression for the equilibrium constant \(K_c\)?
▶️ Answer/Explanation
Ans: C
The equilibrium constant \(K_c\) for the reaction \(H_2(g) + I_2(g) \rightleftharpoons 2HI(g)\) is given by:
\[ K_c = \frac{[HI]^2}{[H_2][I_2]} \]
At equilibrium, 0.26 mol of \(HI\) is formed. Since the stoichiometry shows that 2 moles of \(HI\) are produced per mole of \(H_2\) and \(I_2\) consumed, the changes in concentrations are:
\[ \text{Change in } H_2 = \text{Change in } I_2 = \frac{0.26}{2} = 0.13 \text{ mol} \]
Thus, the equilibrium amounts are:
\[ [H_2] = 0.20 – 0.13 = 0.07 \text{ mol}, \quad [I_2] = 0.15 – 0.13 = 0.02 \text{ mol} \]
Substituting into the \(K_c\) expression:
\[ K_c = \frac{(0.26)^2}{0.07 \times 0.02} \]
Hence, the correct answer is C.
