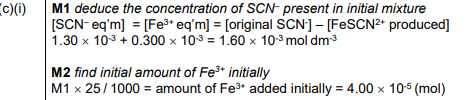(a) Define Le Chatelier’s principle.
(b) Reaction 1 describes the reversible reaction between yellow Fe³⁺(aq) and colourless SCN⁻(aq) to produce red FeSCN²⁺(aq).
reaction 1 Fe³⁺(aq) + SCN⁻(aq) \( \rightleftharpoons \)FeSCN²⁺(aq) ∆H = –x kJmol⁻¹
yellow colourless red
A mixture of Fe³⁺(aq), SCN⁻(aq), and FeSCN²⁺(aq) is at equilibrium at 20°C. The temperature of this mixture is then increased to 50°C and allowed to reach equilibrium. Deduce the changes that occur, if any, in the equilibrium mixture at 50°C compared to the equilibrium mixture at 20°C.
• change in appearance
• change in relative concentration of FeSCN²⁺(aq)
• change in value of the equilibrium constant, \(K_c\)
(c) In another experiment, equimolar amounts of Fe³⁺(aq) and SCN⁻(aq) are mixed together and allowed to reach equilibrium. The total volume of the mixture is 25.0 cm³.
reaction 1 Fe³⁺(aq) + SCN⁻(aq) \( \rightleftharpoons \) FeSCN²⁺(aq)
At equilibrium the mixture contains:
• [SCN⁻] = \(1.30 × 10^{–3}moldm^{–3}\).
• [FeSCN²⁺] = \(0.300 × 10^{–3}moldm^{–3}\).
(i) Calculate the initial amount, in mol, of Fe³⁺(aq) added to SCN⁻(aq) to produce this mixture.
(ii) Calculate \(K_c\) for reaction 1 and state its units. Show your working.
▶️ Answer/Explanation
(a) Le Chatelier’s principle states that if a system at equilibrium is subjected to a change in conditions, the equilibrium will shift to counteract the change and restore equilibrium.
Explanation: This principle helps predict the direction of shift in equilibrium when factors like concentration, temperature, or pressure are altered.
(b)
• Change in appearance: The mixture becomes paler red/more yellow as the equilibrium shifts left (endothermic direction).
• Change in [FeSCN²⁺(aq)]: The concentration of FeSCN²⁺ decreases because the reverse reaction is favored at higher temperatures.
• Change in \(K_c\): The equilibrium constant decreases because the reaction is exothermic (\(\Delta H = -x\)), and increasing temperature favors the reactants.
Explanation: Since the reaction is exothermic, increasing the temperature shifts the equilibrium to the left, reducing FeSCN²⁺ concentration and \(K_c\).
(c)(i) Initial amount of Fe³⁺(aq): \(1.60 \times 10^{-5} \, \text{mol}\).
Explanation: At equilibrium, [SCN⁻] = \(1.30 \times 10^{-3} \, \text{moldm}^{-3}\) and [FeSCN²⁺] = \(0.300 \times 10^{-3} \, \text{moldm}^{-3}\). The initial [SCN⁻] = equilibrium [SCN⁻] + [FeSCN²⁺] = \(1.60 \times 10^{-3} \, \text{moldm}^{-3}\). For 25.0 cm³, the amount is \(1.60 \times 10^{-3} \times 0.025 = 1.60 \times 10^{-5} \, \text{mol}\).
(c)(ii) \(K_c = 178 \, \text{mol}^{-1} \, \text{dm}^3\).
Explanation: Using \(K_c = \frac{[\text{FeSCN}^{2+}]}{[\text{Fe}^{3+}][\text{SCN}^-]}\), and substituting equilibrium concentrations: \(K_c = \frac{0.300 \times 10^{-3}}{(1.30 \times 10^{-3})(1.30 \times 10^{-3})} = 178 \, \text{mol}^{-1} \, \text{dm}^3\).