Lithium reacts with nitrogen at room temperature to form solid Li₃N. Three vessels of equal volume are connected by taps 1 and 2 as shown.
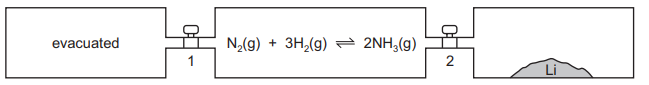
At the start, taps 1 and 2 are closed, the left-hand vessel is evacuated, the middle vessel has the indicated reaction at equilibrium and the right-hand vessel contains lithium only. Which action would allow the equilibrium mixture to contain the most ammonia?
▶️ Answer/Explanation
Ans: A
To maximize ammonia (\(NH_3\)) in the equilibrium mixture, the system should remain undisturbed. Opening any tap would introduce changes (e.g., adding lithium or altering pressure), shifting the equilibrium away from ammonia production. Keeping both taps closed maintains the equilibrium conditions, ensuring the highest ammonia concentration.
When 0.20 mol of hydrogen gas and 0.15 mol of iodine gas are heated at 723K until equilibrium is established, the equilibrium mixture contains 0.26 mol of hydrogen iodide. The equation for the reaction is as follows.
\(H_2(g) + I_2(g) \to 2HI(g)\)
What is the correct expression for the equilibrium constant \(K_c\)?
▶️ Answer/Explanation
Ans: C
1. **Write the equilibrium expression:** For the reaction \(H_2(g) + I_2(g) \rightleftharpoons 2HI(g)\), \[ K_c = \frac{[HI]^2}{[H_2][I_2]} \]
2. **Calculate equilibrium concentrations:** – Since 0.26 mol of HI is formed, the change in \(H_2\) and \(I_2\) is \( \frac{0.26}{2} = 0.13 \) mol (due to stoichiometry). – Equilibrium moles: \[ [H_2] = 0.20 – 0.13 = 0.07 \, \text{mol} \] \[ [I_2] = 0.15 – 0.13 = 0.02 \, \text{mol} \] \[ [HI] = 0.26 \, \text{mol} \]
3. **Substitute into \(K_c\) expression:** \[ K_c = \frac{(0.26)^2}{0.07 \times 0.02} \]
In acidic conditions, iodine reacts with propanone in a substitution reaction.
CH₃COCH₃(aq) + I₂(aq) → CH₃COCH₂I(aq) + HI(aq)
The kinetics of the reaction are investigated using a colorimeter. As the I₂ reacts, the yellow/brown colour of the I₂(aq) fades to colourless, changing the absorbance of the solution. Known concentrations of I₂(aq) are used to prepare a calibration curve graph and the absorbance is then measured as the reaction proceeds.

What is the rate of reaction at 20 s?
▶️ Answer/Explanation
Ans: A
1. Determine the tangent slope at 20 s: The rate of reaction is given by the negative gradient of the concentration-time curve at \( t = 20 \text{ s} \). From the graph, the tangent at 20 s shows a decrease in concentration from approximately \( 0.010 \text{ mol dm}^{-3} \) to \( 0.008 \text{ mol dm}^{-3} \) over 400 s. \[ \text{Rate} = -\frac{\Delta [I₂]}{\Delta t} = -\frac{(0.008 – 0.010)}{400} = 5 \times 10^{-6} \text{ mol dm}^{-3} \text{ s}^{-1} \]
2. Interpret the rate: Since the rate is the change in concentration per unit time, the correct value is \( 5 \times 10^{-6} \text{ mol dm}^{-3} \text{ s}^{-1} \).
Thus, the correct answer is A.
The diagram shows a Boltzmann distribution curve. The axes are not labelled.
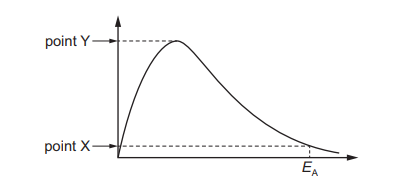
Points X and Y are points on the vertical axis. What is represented by both points X and Y?

▶️ Answer/Explanation
Ans: A
In a Boltzmann distribution curve, the vertical axis represents the number of molecules with a given energy. Points X and Y lie on this axis, indicating they correspond to the number of molecules at specific energy levels. Since the curve shows the distribution of molecular energies, both X and Y represent counts of molecules, making option A (“number of molecules”) correct. Options B, C, and D refer to incorrect interpretations of the axes or the nature of the curve.
