(a) Describe what is meant by dynamic equilibrium.
(b) Reaction 4 describes the reversible reaction between yellow Fe³⁺ (aq) and colourless SCN⁻ (aq) to produce red FeSCN²⁺ (aq).
reaction 4 Fe³⁺(aq) + SCN⁻(aq) \( \rightleftharpoons \) FeSCN²⁺(aq)
yellow colourless red
An equilibrium mixture contains Fe³⁺(aq), SCN⁻ (aq) and FeSCN²⁺ (aq). A few colourless crystals of soluble KSCN(s) are added. The mixture is then left until it reaches equilibrium again. The temperature of both equilibrium mixtures is the same.
(i) Deduce the changes that occur, if any, in the equilibrium mixture after KSCN(s) is added compared to the original equilibrium mixture.
• change in appearance
• change in relative concentration of Fe³⁺(aq)
• change in value of the equilibrium constant, \(K_c\).
(ii) The expression for the equilibrium constant, \(K_c\), for reaction 4 is shown
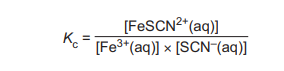
\(5.00 × 10^{–5}mol\) of Fe³⁺(aq) and \(5.00 × 10^{–5}mol\) of SCN⁻ (aq) are added together and allowed to reach equilibrium. The total volume of the mixture is 25.0 cm³. At equilibrium the concentration of FeSCN²⁺(aq) is \(4.23 × 10^{–4}moldm^{–3}\). Calculate the equilibrium constant, \(K_c\), for reaction 4. Include the units in your answer.
(c) Determine the full electronic configuration of Fe³⁺.
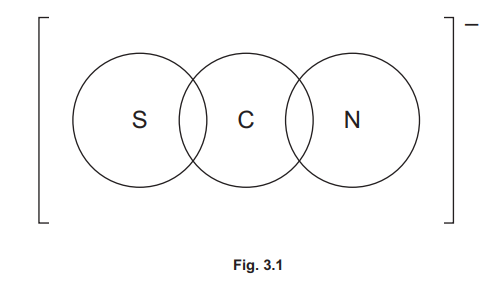
(d) SCN⁻ (aq) is colourless. Complete the dot-and-cross diagram in Fig. 3.1 to show the arrangement of outer electrons in an SCN⁻ ion.
▶️ Answer/Explanation
(a) Dynamic equilibrium occurs when the forward and reverse reaction rates are equal, and the concentrations of reactants and products remain constant over time. This is a state where no macroscopic changes are observed, but molecules continue to react at the molecular level.
Explanation: In dynamic equilibrium, the system is not static—reactants and products are continuously interconverting, but their concentrations do not change because the rates of formation and decomposition are balanced.
(b)(i)
- Change in appearance: The mixture becomes darker red due to increased formation of FeSCN²⁺.
- Change in [Fe³⁺(aq)]: The concentration of Fe³⁺ decreases as more reacts with the added SCN⁻.
- Change in \(K_c\): No change, as \(K_c\) is temperature-dependent and the temperature remains constant.
Explanation: Adding KSCN increases [SCN⁻], shifting the equilibrium to the right (Le Chatelier’s Principle), producing more FeSCN²⁺ and consuming Fe³⁺. \(K_c\) remains unchanged because temperature is constant.
(b)(ii) \(K_c = 1380 \, \text{dm}^3 \text{mol}^{-1}\)
Explanation: First, calculate initial concentrations: \([Fe³⁺]_0 = [SCN⁻]_0 = \frac{5.00 \times 10^{-5} \text{mol}}{0.025 \text{dm}^3} = 2.00 \times 10^{-3} \text{mol dm}^{-3}\). At equilibrium, \([FeSCN²⁺] = 4.23 \times 10^{-4} \text{mol dm}^{-3}\). Using the ICE table, equilibrium concentrations are \([Fe³⁺] = [SCN⁻] = 1.577 \times 10^{-3} \text{mol dm}^{-3}\). Substituting into \(K_c = \frac{[FeSCN²⁺]}{[Fe³⁺][SCN⁻]}\), we get \(K_c = 1380 \, \text{dm}^3 \text{mol}^{-1}\).
(c) \(1s^2 2s^2 2p^6 3s^2 3p^6 3d^5\)
Explanation: Fe has atomic number 26. Fe³⁺ loses 3 electrons (2 from 4s and 1 from 3d), resulting in the configuration \(1s^2 2s^2 2p^6 3s^2 3p^6 3d^5\).
(d)
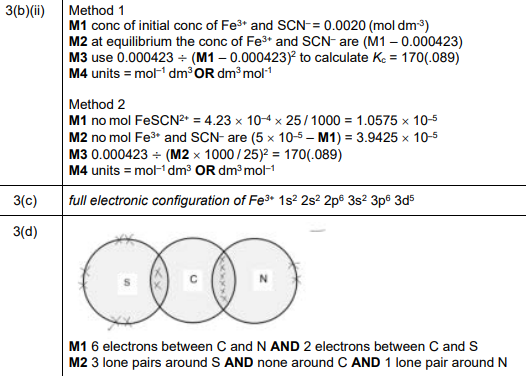
Explanation: The SCN⁻ ion has a lone pair on the sulfur (S), carbon (C), and nitrogen (N) atoms, with covalent bonds between S-C and C-N. The negative charge is delocalized across the ion.
