In alkaline solution, MnO₄⁻ ions oxidise SO₃²⁻ ions to SO₄²⁻ ions. The MnO₄⁻ ions are reduced to MnO₂. What is the ratio of the two ions in the balanced chemical equation for this reaction?

▶️ Answer/Explanation
Ans: A
The reaction involves the oxidation of \(\text{SO}_3^{2-}\) to \(\text{SO}_4^{2-}\) and the reduction of \(\text{MnO}_4^-\) to \(\text{MnO}_2\). Balancing the half-reactions:
Oxidation: \(\text{SO}_3^{2-} + 2\text{OH}^- \rightarrow \text{SO}_4^{2-} + \text{H}_2\text{O} + 2e^-\)
Reduction: \(\text{MnO}_4^- + 2\text{H}_2\text{O} + 3e^- \rightarrow \text{MnO}_2 + 4\text{OH}^-\)
To balance the electrons, multiply the oxidation half-reaction by 3 and the reduction half-reaction by 2. The ratio of \(\text{MnO}_4^-\) to \(\text{SO}_3^{2-}\) in the balanced equation is 2:3 (Option A).
The name ‘chlorate’ is used for an anion consisting of chlorine and oxygen only. In a molecule of ICl, the iodine atom has oxidation number x and the chlorine atom has oxidation number y. When ICl is added to \(H_2O\), iodine is reduced.
\(4ICl + 2H_2O \to 4HCl + O_2 + 2I_2\)
Which statement about the value of x or y is correct?
▶️ Answer/Explanation
Ans: A
Step 1: Determine oxidation numbers in ICl
- In \(ICl\), chlorine is more electronegative, so \(y = -1\).
- Since the molecule is neutral, iodine must have \(x = +1\).
Step 2: Analyze chlorate formation
- Cold NaOH: Forms \(ClO^–\) (hypochlorite), where Cl has an oxidation number of \(+1\).
- Hot NaOH: Forms \(ClO_3^–\) (chlorate), where Cl has an oxidation number of \(+5\).
Step 3: Compare with ICl
Since \(x = +1\) in \(ICl\), it matches the oxidation number of Cl in \(ClO^–\) (formed in cold NaOH). Thus, option A is correct.
Equations for some reactions of hydrogen peroxide are given.
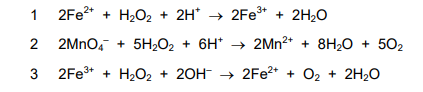
In which reactions is hydrogen peroxide acting as a reducing agent?
▶️ Answer/Explanation
Ans: C
Hydrogen peroxide acts as a reducing agent when it gets oxidized (loses electrons). In reaction 2, the oxygen oxidation state increases from -1 in \( \text{H}_2\text{O}_2 \) to 0 in \( \text{O}_2 \). In reaction 3, it increases from -1 to +2 in \( \text{PbO}_2 \). Reaction 1 shows reduction (acting as oxidizing agent). Thus, the correct answer is C (2 and 3).
A reaction between two gases takes place on the surface of the catalytic converter of a petrol-engined car. In this reaction, four reactant molecules produce three product molecules. What could be the two reactant gases in this reaction?
▶️ Answer/Explanation
Ans: C
The catalytic converter reaction involves the conversion of harmful gases (NO and CO) into less harmful ones (N2 and CO2). The balanced reaction is:
\[ 2NO + 2CO \rightarrow N_2 + 2CO_2 \]
Here, 4 reactant molecules (2NO + 2CO) produce 3 product molecules (1N2 + 2CO2). Thus, the correct answer is C (nitrogen monoxide and carbon monoxide).
