In industry, ethanol is made by reacting ethene with steam in the presence of \( H_3PO_4 \).
reaction 1: \( C_2H_4(g) + H_2O(g) \rightleftharpoons C_2H_5OH(g) \)
(a) Use the bond energy values in Table 4.1 to calculate the enthalpy change, \( \Delta H_r \), for reaction 1.
(b) Reaction 1 reaches equilibrium at constant temperature and pressure.
Deduce what effect increasing the pressure will have on the amount of ethanol in the new equilibrium mixture. Use Le Chatelier’s principle to explain your answer.
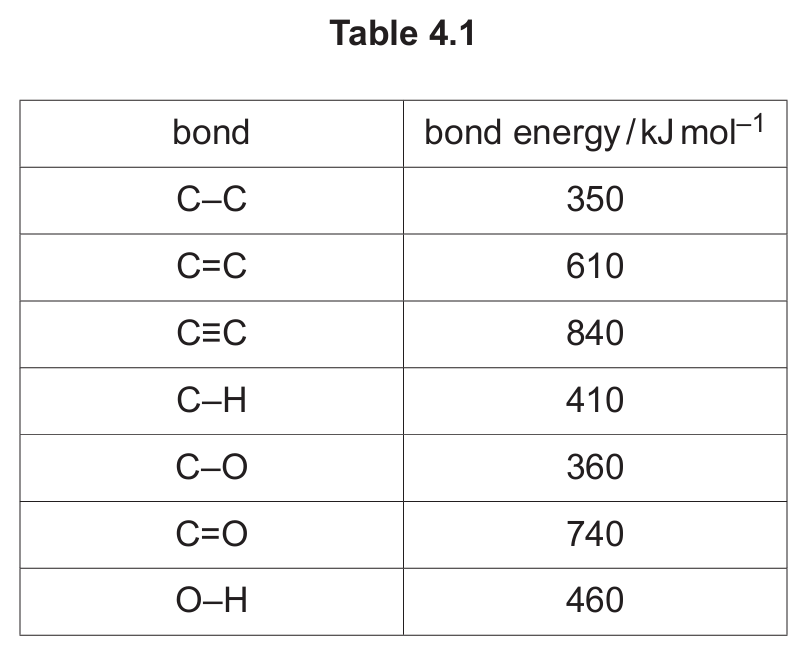
(c) The mechanism for reaction 1 can be described in three steps. Steps 1 and 2 for reaction 1 are shown in Fig. 4.1.
(i) Describe the behaviour of \( H_3PO_4 \) in step 1 in Fig. 4.1. Explain your answer.
(ii) Identify the species that behaves as an electrophile in step 2 in Fig. 4.1. Explain your answer.
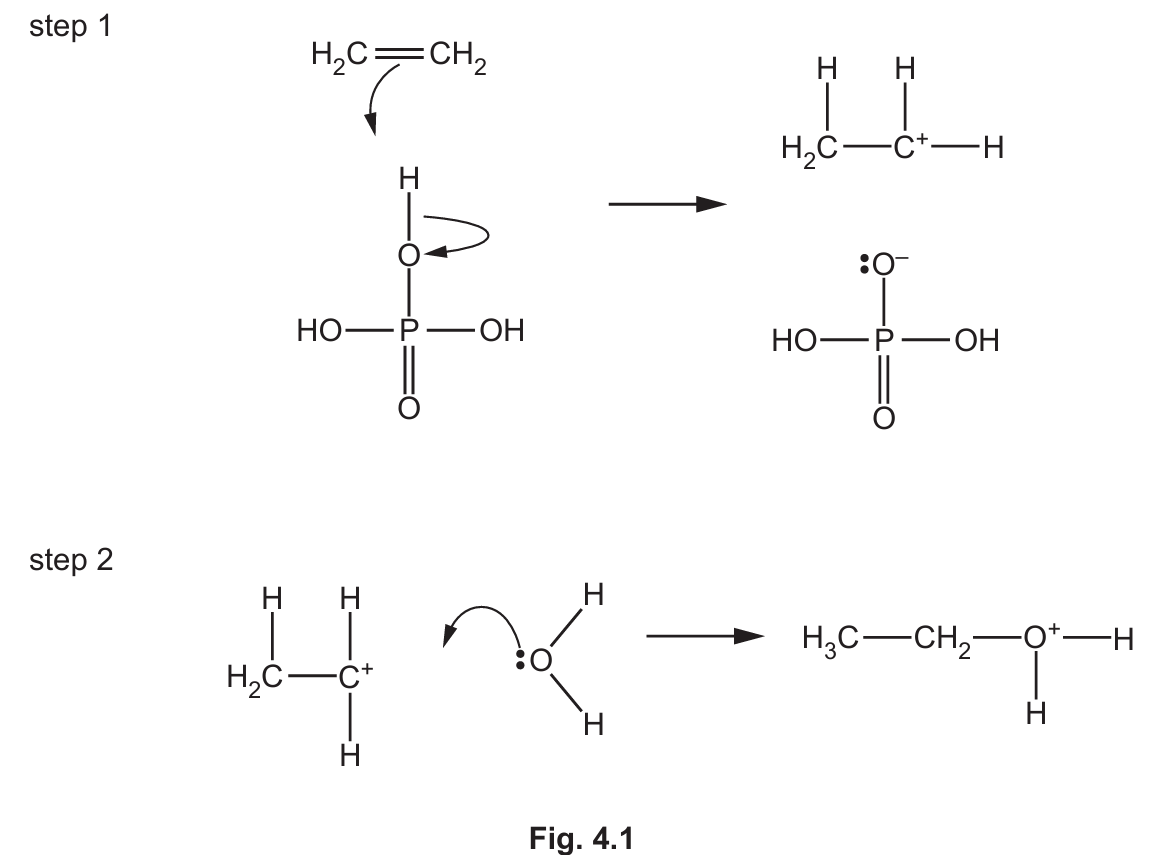
(iii) Complete Fig. 4.2 to show the mechanism for step 3 of reaction 1. Include charges, dipoles, lone pairs of electrons and curly arrows, as appropriate.
(iv) Describe how a catalyst affects a reaction. Explain your answer.
(v) Use Fig. 4.1 and Fig. 4.2 to justify why H3PO4 is described as a catalyst in reaction 1.
(vi) Propene also reacts with steam. A mixture of organic products is produced.
Explain why propan-2-ol is produced in the higher yield.
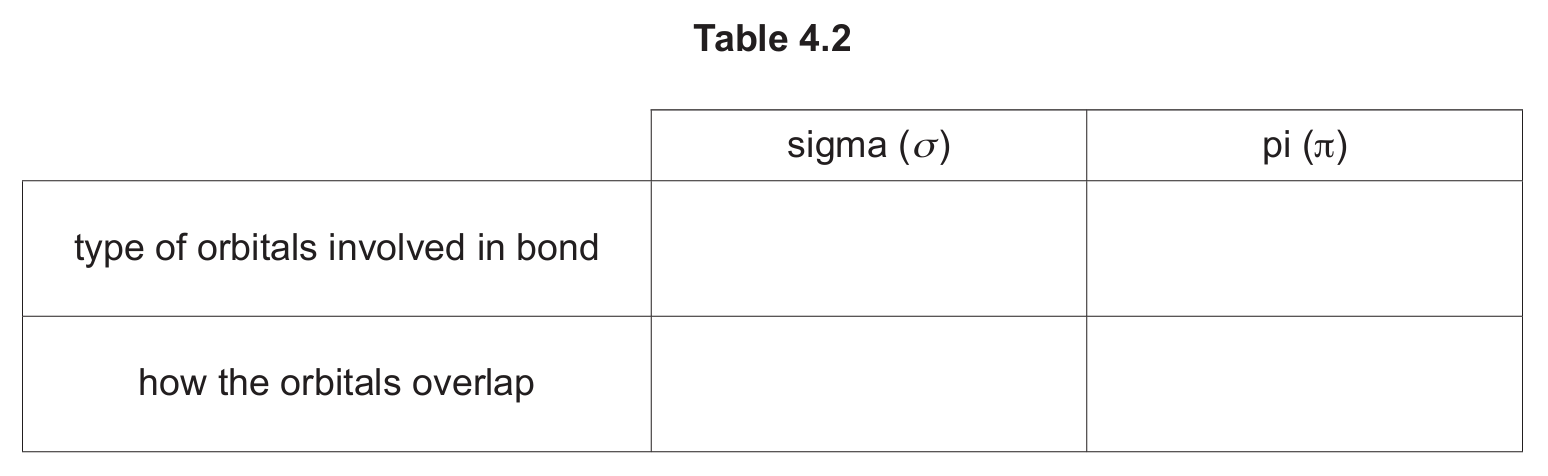
(d) Describe the covalent bonds present between the carbon atoms in an ethene molecule by completing Table 4.2.
▶️ Answer/Explanation
(a) \( \Delta H_r = -50 \, \text{kJ mol}^{-1} \)
Explanation: The enthalpy change for a reaction can be calculated using bond energies. The formula is \( \Delta H = \sum (\text{bond energies of bonds broken}) – \sum (\text{bond energies of bonds formed}) \).
In the reaction \( C_2H_4(g) + H_2O(g) \rightarrow C_2H_5OH(g) \), the bonds broken are: 1 C=C bond (610 kJ/mol), 4 C-H bonds (4 × 410 = 1640 kJ/mol) in ethene, and 2 O-H bonds (2 × 460 = 920 kJ/mol) in water. Total energy absorbed = 610 + 1640 + 920 = 3170 kJ/mol.
The bonds formed in ethanol are: 1 C-C bond (350 kJ/mol), 5 C-H bonds (5 × 410 = 2050 kJ/mol), 1 C-O bond (360 kJ/mol), and 1 O-H bond (460 kJ/mol). Total energy released = 350 + 2050 + 360 + 460 = 3220 kJ/mol.
Therefore, \( \Delta H = 3170 – 3220 = \mathbf{-50 \, \text{kJ mol}^{-1}} \).
(b)
Effect of increasing pressure: The amount of ethanol increases.
Explanation: According to Le Chatelier’s principle, if the pressure on an equilibrium system is increased, the equilibrium will shift to favor the side with fewer moles of gas to reduce the pressure. The reaction is \( C_2H_4(g) + H_2O(g) \rightleftharpoons C_2H_5OH(g) \). The left side has 2 moles of gas (1 + 1), and the right side has 1 mole of gas. Increasing pressure will cause the equilibrium to shift to the right, producing more ethanol.
(c)(i) \( H_3PO_4 \) acts as an acid. It donates a proton (\( H^+ \)).
Explanation: In step 1, phosphoric acid (\( H_3PO_4 \)) donates a proton to the water molecule. This is characteristic acid behavior, as defined by the Brønsted-Lowry theory.
(c)(ii) The species that behaves as an electrophile is \( CH_3CH_2^+ \) (the ethyl carbocation).
Explanation: An electrophile is an electron-deficient species that accepts a pair of electrons. The ethyl carbocation (\( CH_3CH_2^+ \)) has a positively charged carbon atom that is electron-deficient and can accept a lone pair of electrons from a nucleophile.
(c)(iii) The completed mechanism for step 3 should show:
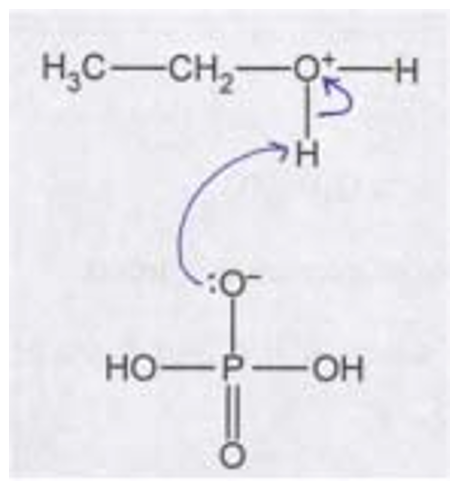
- A curly arrow starting from the lone pair on the oxygen atom of the \( H_2PO_4^- \) ion and going towards the hydrogen atom attached to the oxygen in the protonated ethanol (\( CH_3CH_2OH_2^+ \)).
- A curly arrow showing the breaking of the O-H bond in \( CH_3CH_2OH_2^+ \), with the electrons going to the oxygen atom.
- The products are ethanol (\( CH_3CH_2OH \)) and regenerated phosphoric acid (\( H_3PO_4 \)).
(c)(iv) A catalyst increases the rate of a chemical reaction. It does this by providing an alternative reaction pathway with a lower activation energy.
Explanation: Catalysts work by allowing the reaction to proceed through a different mechanism that requires less energy to get started (lower activation energy). They are not consumed in the overall reaction.
(c)(v) \( H_3PO_4 \) is described as a catalyst because it is regenerated at the end of the reaction. Looking at the steps, it is used in step 1 and then reformed in step 3.
Explanation: A catalyst is a substance that speeds up a reaction without being permanently changed. Since \( H_3PO_4 \) is not used up overall (it is reformed), it qualifies as a catalyst.
(c)(vi) Propan-2-ol is produced in higher yield because its formation proceeds via a more stable secondary carbocation intermediate.
Explanation: When propene (\( CH_3CH=CH_2 \)) reacts with steam and an acid catalyst, it can form two carbocations: a primary carbocation (\( CH_3CH_2CH_2^+ \)) or a more stable secondary carbocation (\( (CH_3)_2CH^+ \)). The secondary carbocation is more stable due to the positive inductive effect of the two alkyl (methyl) groups, which donate electrons towards the positive charge. This greater stability means the secondary carbocation forms more easily and leads to a higher yield of the product derived from it, which is propan-2-ol.
(d)
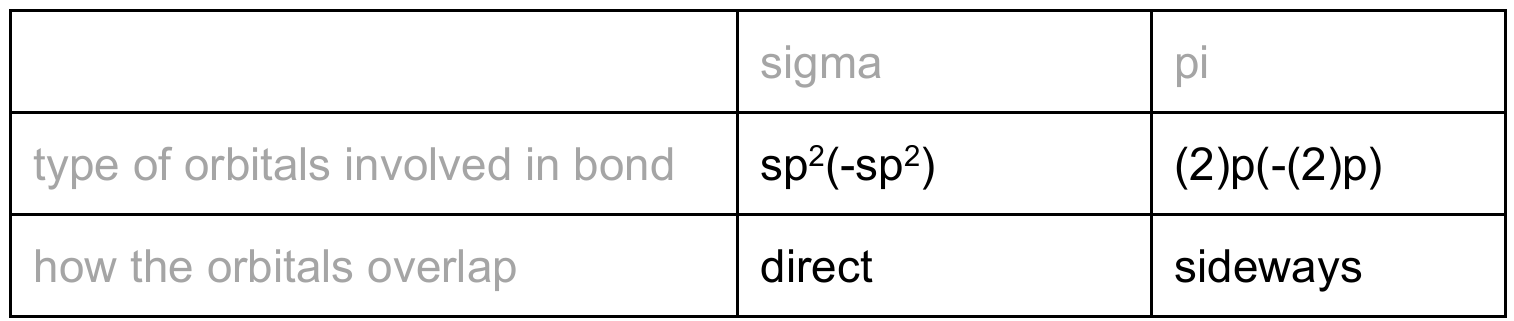
Explanation: In ethene (\( C_2H_4 \)), the carbon atoms are sp² hybridized. The sigma (σ) bond between the carbons is formed by the direct, end-to-end overlap of two sp² hybrid orbitals, one from each carbon atom. The pi (π) bond is formed by the sideways overlap of two unhybridized p orbitals, one from each carbon atom, which are perpendicular to the plane of the molecule.
