Vanadium, niobium and tantalum are metals in the same group of the Periodic Table.
(a) The shorthand electronic configuration of vanadium in the ground state is \([Ar]3d^34s^2\).
(i) State what is meant by the term ground state.
(ii) Show the electronic configuration of vanadium using electrons in boxes notation.
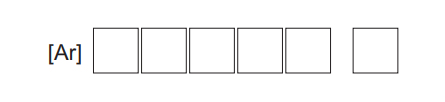
(iii) Deduce the total number of electrons in the p sub-shells of a vanadium atom.
(b) Pelopium was the suggested name for a new element discovered in a mineral. Pelopium was later found to be a mixture of niobium, Nb, and tantalum, Ta. Only one naturally occurring isotope exists for each of Nb and Ta.
(i) Complete Table 1.1.
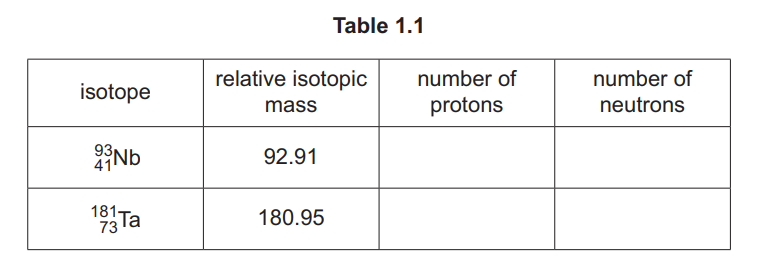
(ii) Define relative isotopic mass.
(iii) A sample of pelopium contains 90.9% by mass \(^{93}_{41}Nb\) and 9.1% by mass \(^{181}_{73}Ta\). Calculate the theoretical relative atomic mass of pelopium based on these data and Table 1.1. Give your answer to two decimal places. Show your working.
▶️ Answer/Explanation
(a)(i) The ground state refers to the lowest energy state of an atom where all electrons occupy the lowest available orbitals.
(a)(ii) The electronic configuration of vanadium in boxes notation is:
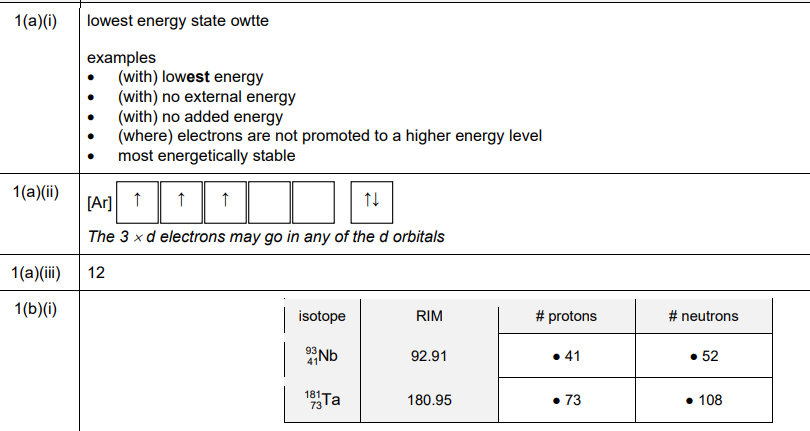
(a)(iii) Vanadium has 12 electrons in the p sub-shells (from the argon core \([Ar]\), which includes \(2p^6\) and \(3p^6\)).
(b)(i) The completed Table 1.1 is:

(b)(ii) Relative isotopic mass is the mass of an isotope relative to \(\frac{1}{12}\) the mass of a carbon-12 atom.
(b)(iii) The relative atomic mass of pelopium is calculated as:
\( (92.91 \times 0.909) + (180.95 \times 0.091) = 84.46 + 16.46 = 100.92 \).
Final Answer: \(\boxed{100.92}\)
