Which alkene shows geometric isomerism?

▶️ Answer/Explanation
Ans: B
Geometric (cis-trans) isomerism occurs in alkenes when each carbon of the double bond has two different groups attached. In the given options:
- Option B has the structure \(\text{CH}_3\text{CH=CHCH}_3\) (2-butene), where each double-bonded carbon is attached to a hydrogen and a methyl group (\(\text{-CH}_3\)), allowing for cis and trans forms.
- Other options either have identical groups on one carbon (no isomerism) or are symmetrical.
Thus, B is the correct answer.
Catalytic converters are fitted in the exhaust systems of many cars.
Gas X:
● causes acid rain if it is released into the air
● is removed from car exhaust fumes by a catalytic converter.
What is gas X?
A. carbon dioxide
B. carbon monoxide
C. hydrocarbon vapour
D. nitrogen dioxide
▶️ Answer/Explanation
Ans: D
Explanation:
- Acid Rain Contribution:
- Nitrogen dioxide (\(NO_2\)) reacts with water vapor in the atmosphere to form nitric acid (\(HNO_3\)), a major contributor to acid rain.
- Carbon dioxide (\(CO_2\)) contributes weakly to acid rain (carbonic acid), but it is not a primary target of catalytic converters.
- Role of Catalytic Converters:
- Catalytic converters primarily reduce harmful emissions by converting:
- Nitrogen oxides (\(NO_x\), including \(NO_2\)) into \(N_2\) and \(O_2\).
- Carbon monoxide (\(CO\)) into \(CO_2\).
- Unburned hydrocarbons into \(CO_2\) and \(H_2O\).
- While \(CO\) and hydrocarbons are also removed, only \(NO_2\) directly causes acid rain among the options.
- Catalytic converters primarily reduce harmful emissions by converting:
Thus, Gas X is nitrogen dioxide (\(NO_2\)), making the correct answer D.
The diagram shows the structure of the naturally occurring molecule cholesterol.
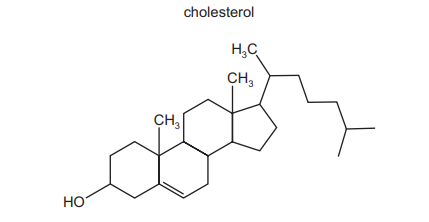
Student X stated that the 17 carbon atoms in the 4 rings all lie in the same plane. Student Y stated that this molecule displays cis/trans isomerism at the C=C double bond. Which students are correct?
▶️ Answer/Explanation
Ans: B
Analysis of Student X’s claim: The four rings in cholesterol are cyclohexane (chair conformation) and cyclopentane (envelope conformation), which are non-planar due to their 3D geometry. Thus, the 17 carbon atoms cannot lie in the same plane.
Analysis of Student Y’s claim: The C=C bond (between C5 and C6) has two identical hydrogen atoms on one carbon, making cis/trans isomerism impossible (both substituents on C5 are H atoms).
Since both statements are incorrect, the correct answer is B (neither X nor Y).
Compound Q can be hydrolysed by HCl(aq). The two products of this hydrolysis have the same empirical formula.
What could Q be?
▶️ Answer/Explanation
Ans: B
Hydrolysis of diester B (\( CH_3CO_2CH_2CH_2CO_2H \)) gives HOCH₂CH₂OH (ethanediol) and CH₃CO₂H (ethanoic acid). Both products have the empirical formula CH₂O. The others do not yield two products with identical empirical formulas.
