Question
(a) A nucleus of sodium-22 ![]() decays by emitting a \(β^+\) particle. A different nucleus is formed by the decay.
decays by emitting a \(β^+\) particle. A different nucleus is formed by the decay.
(i) State the name of another lepton that is produced by the decay.
…………………………………………………………….. [1]
(ii) Determine the nucleon number and the proton number of the nucleus that is formed by the decay.
nucleon number = ………………………………………………………
proton number = ………………………………………………………
[2]
(iii) The quark composition of a nucleon in the sodium-22 nucleus is changed during the decay.
Describe the change to the quark composition of the nucleon.
………………………………………………………………………………
……………………………………………………………………………………… [1]
(b) A baryon consists of quarks that are the same flavour (type). The charge of the baryon is –2e, where e is the elementary charge.
(i) Calculate, in terms of e, the charge of each quark.
charge = ……………………………………………… e [1]
(ii) State a possible flavour (type) of the quarks.
………………………………………………………. [1]
[Total: 6]
Answer/Explanation
Ans:
(a)(i) (electron) neutrino
(a)(ii) nucleon number = 22
proton number = 10
(a)(iii) up up down changes to up down down or up changes to down
(b)(i) charge = – 2⁄3e
(b)(ii) antiup / anticharm / antitop
Question
(a) The results of the α-particle scattering experiment provide evidence for the structure of the
atom.
Result 1: The vast majority of the α-particles pass straight through the metal foil or are
deviated by small angles.
Result 2: A very small minority of α-particles is scattered through angles greater than 90°.
State what may be inferred (deduced) from:
(i) result 1 [1]
(ii) result 2. [2]
(b) A radioactive decay sequence contains four nuclei, P, Q, R and S, as shown.
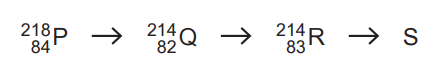
Nucleus S is an isotope of nucleus P.
(i) Determine the proton number and the nucleon number of nucleus S.
proton number = ………………………………………………………
nucleon number = ……………………………………………………… [2]
(ii) The quark composition of a nucleon in Q changes as Q decays to form R.
Describe this change to the quark composition of the nucleon. [1]
[Total: 6]
Answer/Explanation
Ans
(a) (i) most of the atom is empty space
or
the nucleus (volume) is very small compared to the atom
(a) (ii) the nucleus is charged
the mass is concentrated in nucleus / small region / small volume / small core
or
the majority of the mass is in nucleus / small region / small volume / small core
(b) (i) proton number = 84
nucleon number = 214
(b) (ii) up down down changes to up up down / udd → uud
or
down changes to up / d → u
