Question
(a) State
(i)what may be deduced from the difference in the temperatures of two objects,
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
(ii) the basic principle by which temperature is measured.
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
(b) By reference to your answer in (a)(ii), explain why two thermometers may not give the same temperature reading for an object.
…………………………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………………………
(c) A block of aluminium of mass 670g is heated at a constant rate of 95W for 6.0 minutes.
The specific heat capacity of aluminium is 910Jkg−1K−1.
The initial temperature of the block is 24°C.
(i) Assuming that no thermal energy is lost to the surroundings, show that the final temperature of the block is 80°C.
(ii) In practice, there are energy losses to the surroundings.
The actual variation with time t of the temperature θ of the block is shown in Fig. 1.1.
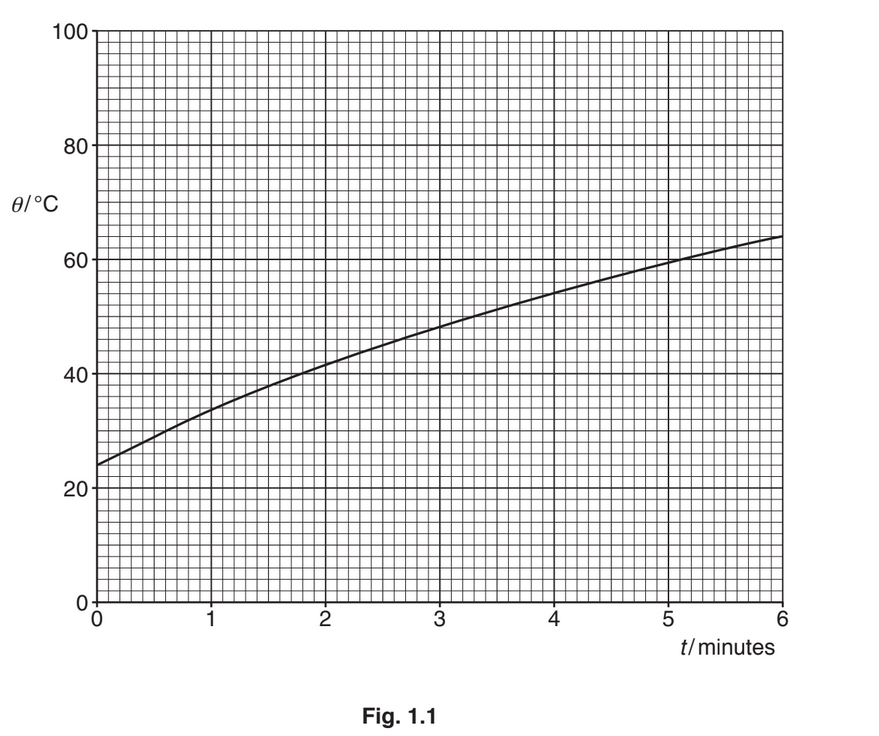
1. Use the information in (i) to draw, on Fig. 1.1, a line to represent the temperature of the block, assuming no energy losses to the surroundings.
2. Using Fig. 1.1, calculate the total energy loss to the surroundings during the heating process.
energy loss = ……………………………………………… J
Answer/Explanation
a)(i)
direction or rate of transfer of (thermal) energy
or
(if different,) not in thermal equilibrium/energy is transferred
(a)(ii)
uses a property (of a substance) that changes with temperature
(b)(i)
• temperature scale assumes linear change of property with temperature
• physical properties may not vary linearly with temperature
• agrees only at fixed points
Any 2 points.
(b)(ii)
1. sketch: straight line from (0,24) to (6,80)
2. temperature drop due to energy loss = (80 – 64) = 16°C
energy loss = 0.670 × 910 × (80 – 64) = 9800J
or
energy to raise temperature to 64°C = 0.670 × 910 × (64 – 24)
= 24400J
Question
(a) Two metal spheres are in thermal equilibrium. State and explain what is meant by thermal equilibrium.
(b)An electric water heater contains a tube through which water flows at a constant rate. The water in the tube passes over a heating coil, as shown in Fig. 3.1.
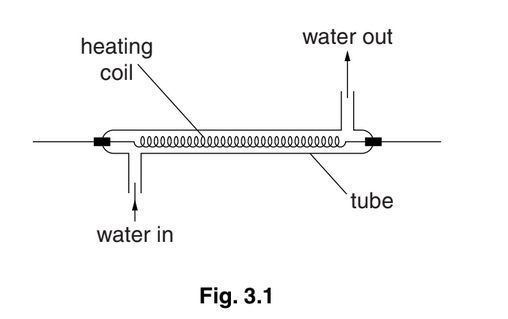
The water flows into the tube at a temperature of 18 °C. When the power of the heater is 3.8 kW, the temperature of the water at the outlet is 42 °C. The specific heat capacity of water is \(4.3J~g^{-1}K^{-1}\)
\(flow rate = …………………………………. gs^{-1})
(ii) State and explain whether your answer in (i) is likely to be an overestimate or an underestimate of the flow rate
Answer/Explanation
(a) temperature of the spheres is the same no (net) transfer of energy between the spheres
(b) (i) \(power=m\times c\times \Delta \theta\) where m is mass per second
\(3800=m\times 4.2\times (42-18)\)
\(m=28~gs^{-1}\)
(ii)
