Question
A fixed mass of an ideal gas has a volume V and a pressure p. The gas undergoes a cycle of changes, X to Y to Z to X, as shown in Fig. 2.1.
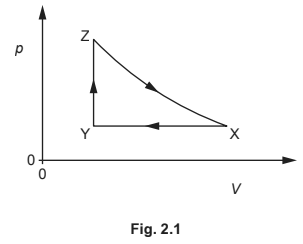
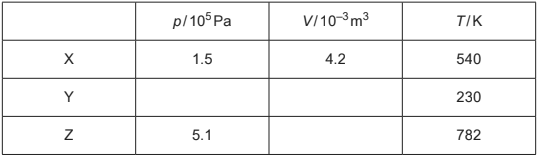
Table 2.1 shows data for p, V and temperature T for the gas at points X, Y and Z.
Table 2.1
(a) State the change in internal energy ΔU for one complete cycle, XYZX.
(b) Calculate the amount n of gas.
(c) Complete Table 2.1.
Use the space below for any working.
(d) (i) The first law of thermodynamics for a system may be represented by the equation
ΔU = q + W.
State, with reference to the system, what is meant by:
(ii) Explain how the first law of thermodynamics applies to the change Z to X.
Answer/Explanation
Ans:
(a) \(0\)
(b) \(pV\) = \(nRT\)
\((n =) 1.5 \times 10^{5} \times 4.2 \times 10^{–3} / 8.31 \times 540\)
= 0.14 mol
(c) missing pressure \(1.5 ( \times 10^{5})\)
both missing volumes \(1.8 ( \times 10^{–3})\)
(d) (i) \((ΔU:)\) increase in internal energy (of the system)
\((q:)\) thermal energy supplied to the system B1
\((W:)\) work done on system
(ii) volume increases and work is done by the gas
Question
(a) Define specific heat capacity. [2]
(b) A sealed container of fixed volume V contains N molecules, each of mass m, of an ideal gas at pressure p.
(i) State an expression, in terms of V, N, p and the Boltzmann constant k, for the thermodynamic temperature T of the gas. [1]
(ii) Show that the mean translational kinetic energy \(E_K\) of a molecule of the gas is given by
\(E_{k}=\frac{3}{2}kT\) [2]
(iii) Explain why the internal energy of the gas is equal to the total kinetic energy of the molecules. [2]
(c) The gas in (b) is supplied with thermal energy Q.
(i) Explain, with reference to the first law of thermodynamics, why the increase in internal energy of the gas is Q. [2]
(ii) Use the expression in (b)(ii) and the information in (c)(i) to show that the specific heat capacity c of the gas is given by
\(c=\frac{3k}{2m}\) [2]
(d) The container in (b) is now replaced with one that does not have a fixed volume. Instead, the gas is able to expand, so that the pressure of the gas remains constant as thermal energy is supplied. Suggest, with a reason, how the specific heat capacity of the gas would now compare with the value in (c)(ii). [2 [Total: 13]
Answer/Explanation
Ans
(a) (thermal) energy per unit mass (to cause temperature change)
(thermal) energy per unit change in temperature B1
(b) (i) (T =) pV / Nk
(b) (ii) (pV =) NkT = ⅓Nm < c2>
or
pV = NkT and pV = ⅓Nm < c2>
leading to ½ m <c2> = (3/2)kT and ½ m <c2> = EK
(b) (iii) internal energy = Σ EK (of molecules) + Σ EP (of molecules)
or
no forces between molecules
potential energy of molecules is zero
(c) (i) increase in internal energy = Q + work done
constant volume so no work done
(c) (ii) c = Q / Nm ΔT
= [N × (3/2)kΔT] / (NmΔT) = 3k /2m
(d) (as it expands) gas does work (against the atmosphere/external pressure) B1
