AS Physics Errors and uncertainties Study Notes
AS Physics Errors and uncertainties Study Notes
AS Physics Errors and uncertainties Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS Physics Study Notes syllabus with Candidates should be able to:
- understand and explain the effects of systematic errors (including zero errors) and random errors in measurements
- understand the distinction between precision and accuracy
- assess the uncertainty in a derived quantity by simple addition of absolute or percentage uncertainties
Systematic and Random Errors in Measurements
All measurements in physics are subject to some form of error the difference between the measured value and the true value of a physical quantity. Understanding and minimizing these errors is essential to improve the accuracy and reliability of experimental results.
Types of Errors in Measurement:
| Error Type | Definition | Cause | Effect on Results | Reduction / Correction |
|---|---|---|---|---|
| Systematic Error | Error that causes all measurements to deviate from the true value by a fixed amount in the same direction (either all too high or all too low). | Faulty equipment, incorrect calibration, zero error, or consistent observer bias. | Affects accuracy — shifts all values by the same amount; cannot be reduced by repetition. | Identify and correct source (e.g., recalibrate instrument, apply correction for zero error). |
| Random Error | Error that causes measurements to fluctuate unpredictably around the true value due to uncontrollable variations. | Environmental changes, human reaction time, or limitations of instrument precision. | Affects precision — leads to scatter of readings; averages may still be close to the true value. | Take multiple readings and calculate the mean; use instruments with finer scale divisions. |
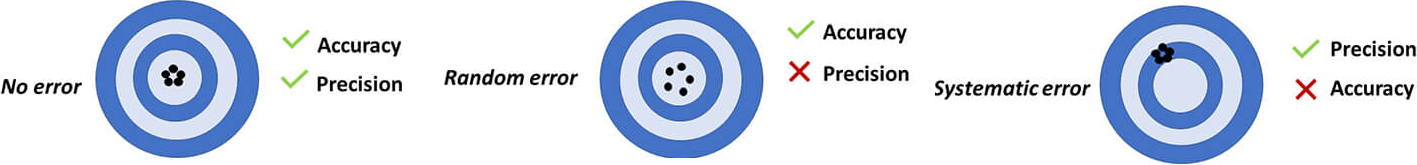
Zero Error (a type of Systematic Error):
- Occurs when an instrument reads a non-zero value even when the measured quantity should be zero.
- Example: A vernier caliper that reads \( \mathrm{+0.02\,mm} \) when fully closed has a +0.02 mm zero error.
- Correction: Subtract or add the zero error value from all readings as appropriate.
Comparison Between Systematic and Random Errors:
| Aspect | Systematic Error | Random Error |
|---|---|---|
| Nature | Consistent deviation in one direction | Unpredictable variations around true value |
| Effect on Accuracy | Decreases accuracy (biases results) | Decreases precision (scatter in data) |
| Detection | Difficult to detect by repetition alone | Detected by irregular fluctuations between readings |
| Reduction Method | Calibration, correction, or elimination of source | Increase number of readings and average |
Example
1. A stopwatch starts \( \mathrm{0.2\,s} \) late every time it is used.
2. A student repeatedly measures the length of a wire and records slightly different values each time. Identify the Error Types
▶️ Answer / Explanation
Case 1: Stopwatch delay = constant shift = Systematic error (zero or calibration error). Correction: Subtract \( \mathrm{0.2\,s} \) from each timing.
Case 2: Small variations between measurements = Random error. Correction: Take multiple readings and average them to reduce effect.
Distinction Between Precision and Accuracy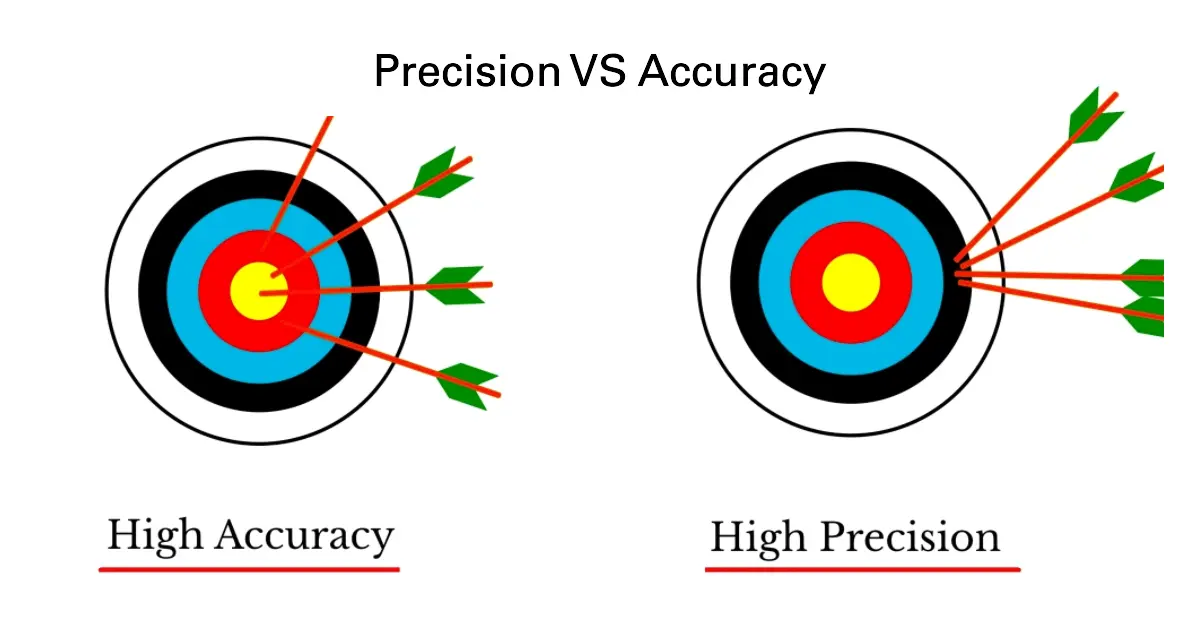
In experimental physics, precision and accuracy describe different aspects of the quality of a measurement. While they are often used together, they do not mean the same thing.
Key Idea:
- Precision is about consistency — how close repeated measurements are to each other.
- Accuracy is about truth — how close a measurement is to the true or accepted value.
Comparison Between Precision and Accuracy:
| Aspect | Precision | Accuracy |
|---|---|---|
| Meaning | How close repeated measurements are to each other. | How close a measurement is to the true or accepted value. |
| Dependence | Affected by random errors. | Affected by systematic errors. |
| Indicator | Small variation or scatter between readings. | Small deviation from the true value. |
| Improved by | Taking repeated readings and averaging results. | Calibrating instruments and correcting systematic errors. |
| Example | Repeated measurements of length are all \( \mathrm{10.02\,cm} \), \( \mathrm{10.03\,cm} \), \( \mathrm{10.02\,cm} \). | Mean measured length \( \mathrm{10.0\,cm} \) is close to the true value \( \mathrm{10.00\,cm} \). |
| Effect on Data Spread | High precision → small spread of readings. | High accuracy → readings centred near the true value. |
Visual Analogy (Conceptual Explanation):
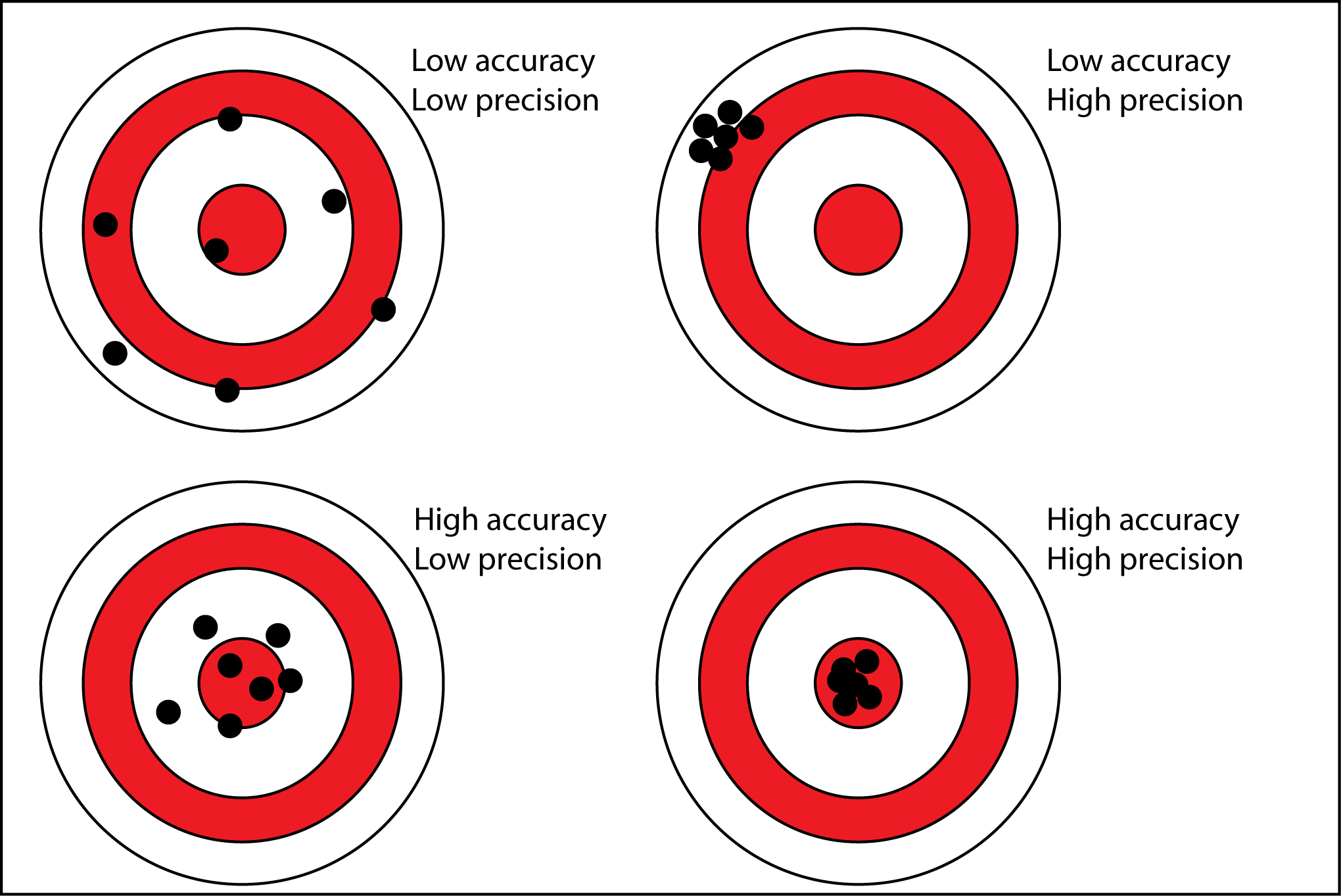
| Condition | Description |
|---|---|
| High Precision, Low Accuracy | All readings are close together but far from the true value (consistent but wrong). |
| Low Precision, High Accuracy | Readings are spread out but the average is close to the true value. |
| High Precision, High Accuracy | All readings are close together and close to the true value (ideal measurement). |
| Low Precision, Low Accuracy | Readings are spread out and far from the true value (poor measurement). |
Example
Understanding Precision vs Accuracy
A student measures the diameter of a wire using a micrometer screw gauge and records:
\( \mathrm{1.23\,mm,\, 1.22\,mm,\, 1.24\,mm,\, 1.23\,mm} \)
The true diameter is \( \mathrm{1.50\,mm} \).
▶️ Answer / Explanation
Step 1: The readings are very close to each other → high precision.
Step 2: However, all are far from the true value → low accuracy.
Conclusion: The instrument may have a systematic error (e.g., zero error). It produces consistent but incorrect readings — precise but not accurate.
Assessing the Uncertainty in a Derived Quantity
Every measurement has an uncertainty a numerical estimate of how much the measured value may differ from the true value. When quantities are combined by mathematical operations, their uncertainties must also be combined appropriately to estimate the overall uncertainty in the derived result.
Key Idea: Uncertainties in addition and subtraction combine by adding absolute uncertainties. Uncertainties in multiplication, division, or powers combine by adding percentage (or fractional) uncertainties.
1. Absolute Uncertainty
- Represents the margin of possible error in a measurement, with the same unit as the measured quantity.
- Example: \( \mathrm{L = (50.0 \pm 0.1)\,cm} \)
- Here, \( \mathrm{0.1\,cm} \) is the absolute uncertainty.
2. Fractional Uncertainty
- Ratio of absolute uncertainty to the measured value:
\( \mathrm{Fractional\ uncertainty = \dfrac{\text{absolute uncertainty}}{\text{measured value}}} \)
Example: \( \mathrm{\dfrac{0.1}{50.0} = 0.002 = 0.2\%} \)
3. Percentage Uncertainty
- Fractional uncertainty expressed as a percentage:
\( \mathrm{Percentage\ uncertainty = \dfrac{\text{absolute uncertainty}}{\text{measured value}} \times 100\%} \)
Rules for Combining Uncertainties:
| Operation | Equation | Rule for Uncertainty | Type of Uncertainty Added |
|---|---|---|---|
| Addition | \( \mathrm{Q = A + B} \) | \( \mathrm{\Delta Q = \Delta A + \Delta B} \) | Absolute |
| Subtraction | \( \mathrm{Q = A – B} \) | \( \mathrm{\Delta Q = \Delta A + \Delta B} \) | Absolute |
| Multiplication | \( \mathrm{Q = A \times B} \) | \( \mathrm{\dfrac{\Delta Q}{Q} = \dfrac{\Delta A}{A} + \dfrac{\Delta B}{B}} \) | Percentage or fractional |
| Division | \( \mathrm{Q = \dfrac{A}{B}} \) | \( \mathrm{\dfrac{\Delta Q}{Q} = \dfrac{\Delta A}{A} + \dfrac{\Delta B}{B}} \) | Percentage or fractional |
| Powers | \( \mathrm{Q = A^n} \) | \( \mathrm{\dfrac{\Delta Q}{Q} = n \dfrac{\Delta A}{A}} \) | Percentage or fractional |
Example
Two lengths are measured as:
\( \mathrm{L_1 = (35.0 \pm 0.1)\,cm, \quad L_2 = (25.0 \pm 0.2)\,cm.} \)
Find the total length and its uncertainty.
▶️ Answer / Explanation
Step 1: Add the measurements.
\( \mathrm{L = L_1 + L_2 = 35.0 + 25.0 = 60.0\,cm} \)
Step 2: Add absolute uncertainties.
\( \mathrm{\Delta L = 0.1 + 0.2 = 0.3\,cm} \)
Result: \( \mathrm{L = (60.0 \pm 0.3)\,cm.} \)
Example
A student measures:
\(\mathrm{V = (2.00 \pm 0.02)\,m/s}, \quad \mathrm{t = (5.00 \pm 0.10)\,s}\)
Find the distance \( \mathrm{s = Vt} \) and its percentage uncertainty.
▶️ Answer / Explanation
Step 1: Calculate distance.
\( \mathrm{s = 2.00 \times 5.00 = 10.0\,m} \)
Step 2: Find percentage uncertainties.
\( \mathrm{\dfrac{\Delta V}{V} = \dfrac{0.02}{2.00} \times 100 = 1.0\%} \)
\( \mathrm{\dfrac{\Delta t}{t} = \dfrac{0.10}{5.00} \times 100 = 2.0\%} \)
Step 3: Add percentage uncertainties.
\( \mathrm{\%\,\Delta s = 1.0 + 2.0 = 3.0\%} \)
Step 4: Find absolute uncertainty in \( \mathrm{s} \).
\( \mathrm{\Delta s = 3\%\ \text{of}\ 10.0 = 0.3\,m} \)
Result: \( \mathrm{s = (10.0 \pm 0.3)\,m.} \)
