CIE AS/A Level Physics 11.1 Atoms, nuclei and radiation Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 11.1 Atoms, nuclei and radiation Study Notes – New Syllabus
CIE AS/A Level Physics 11.1 Atoms, nuclei and radiation Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- infer from the results of the α-particle scattering experiment the existence and small size of the nucleus
- describe a simple model for the nuclear atom to include protons, neutrons and orbital electrons
- distinguish between nucleon number and proton number
- understand that isotopes are forms of the same element with different numbers of neutrons in their nuclei
- understand and use the notation \(^A_ZX \) for the representation of nuclides
- understand that nucleon number and charge are conserved in nuclear processes
- describe the composition, mass and charge of α-, β- and γ-radiations (both β⁻ (electrons) and β⁺ (positrons) are included)
- understand that an antiparticle has the same mass but opposite charge to the corresponding particle, and that a positron is the antiparticle of an electron
- state that (electron) antineutrinos are produced during β⁻ decay and (electron) neutrinos are produced during β⁺ decay
- understand that α-particles have discrete energies but that β-particles have a continuous range of energies because (anti)neutrinos are emitted in β-decay
- represent α- and β-decay by a radioactive decay equation of the form\(^{238}_{92}\mathrm{U} \rightarrow ^{234}_{90}\mathrm{Th} + ^4_{2}\alpha\)
- use the unified atomic mass unit (u) as a unit of mass
Inference from the α-Particle Scattering Experiment — Existence and Small Size of the Nucleus
In 1909, Ernest Rutherford and his team (Geiger and Marsden) performed the famous α-particle scattering experiment to investigate the structure of the atom. They directed a beam of positively charged alpha particles (\( \mathrm{He^{2+}} \)) at a very thin sheet of gold foil (~1 atom thick).![]()
Observations:
- Most alpha particles passed straight through the foil without any deflection.
- Some were deflected through small angles.
- A very few (about 1 in 10,000) were deflected back through angles greater than 90° — some even rebounded.
Conclusions (Inferences):
- Most of the atom is empty space since most α-particles passed through undeflected.
- All the positive charge and most of the mass are concentrated in a tiny central region — the nucleus.
- The nucleus is very small compared to the size of the atom (about 1/10,000th the atom’s radius).
- Strong repulsion during large deflections indicates that the nucleus is positively charged.
Rutherford’s α-scattering experiment proved the atom has a small, dense, positively charged nucleus surrounded by mostly empty space.
Example
Why did most of the alpha particles pass through the gold foil undeflected in Rutherford’s experiment?
▶️ Answer / Explanation
Most alpha particles passed through undeflected because atoms are mostly empty space. The alpha particles only occasionally come close enough to the dense, positively charged nucleus to be deflected.
The Simple Model of the Nuclear Atom
Rutherford’s Nuclear Model: Based on the scattering experiment, Rutherford proposed the nuclear model of the atom — which replaced the earlier “plum pudding model” by J.J. Thomson.
Structure of the Atom (Modern View):![]()
- An atom consists of a tiny central nucleus containing protons and neutrons.
- The nucleus contains almost all of the atom’s mass.
- Electrons orbit the nucleus at relatively large distances, occupying energy levels or shells.
- The atom is electrically neutral because the number of protons equals the number of electrons.
- The size of the atom (~\( \mathrm{10^{-10} \ m} \)) is much larger than the nucleus (~\( \mathrm{10^{-15} \ m} \)).
Diagrammatic Representation:
Key Particles of the Atom:
| Particle | Symbol | Charge | Relative Mass | Location |
|---|---|---|---|---|
| Proton | \( \mathrm{p} \) | \( \mathrm{+1e} \) | 1 | Nucleus |
| Neutron | \( \mathrm{n} \) | 0 | 1 | Nucleus |
| Electron | \( \mathrm{e^-} \) | \( \mathrm{-1e} \) | 1/1836 | Orbiting around nucleus in shells |
The nuclear atom consists of a dense, positively charged nucleus (protons and neutrons) surrounded by electrons in orbits — most of the atom’s volume is empty space.
Example
Why does almost all the mass of an atom lie in the nucleus?
▶️ Answer / Explanation
Protons and neutrons, which are located in the nucleus, are about 2000 times more massive than electrons. Since electrons have negligible mass, almost all the atom’s mass is concentrated in the nucleus.
Distinguishing Between Nucleon Number and Proton Number
Definitions:
| Quantity | Symbol | Definition | Example (Carbon-12) |
|---|---|---|---|
| Proton number (Atomic number) | \( \mathrm{Z} \) | Number of protons in the nucleus of an atom. It determines the element’s identity and its position in the periodic table. | \( \mathrm{Z = 6} \) |
| Nucleon number (Mass number) | \( \mathrm{A} \) | Total number of protons and neutrons (nucleons) in the nucleus. | \( \mathrm{A = 12} \) |
Relationship: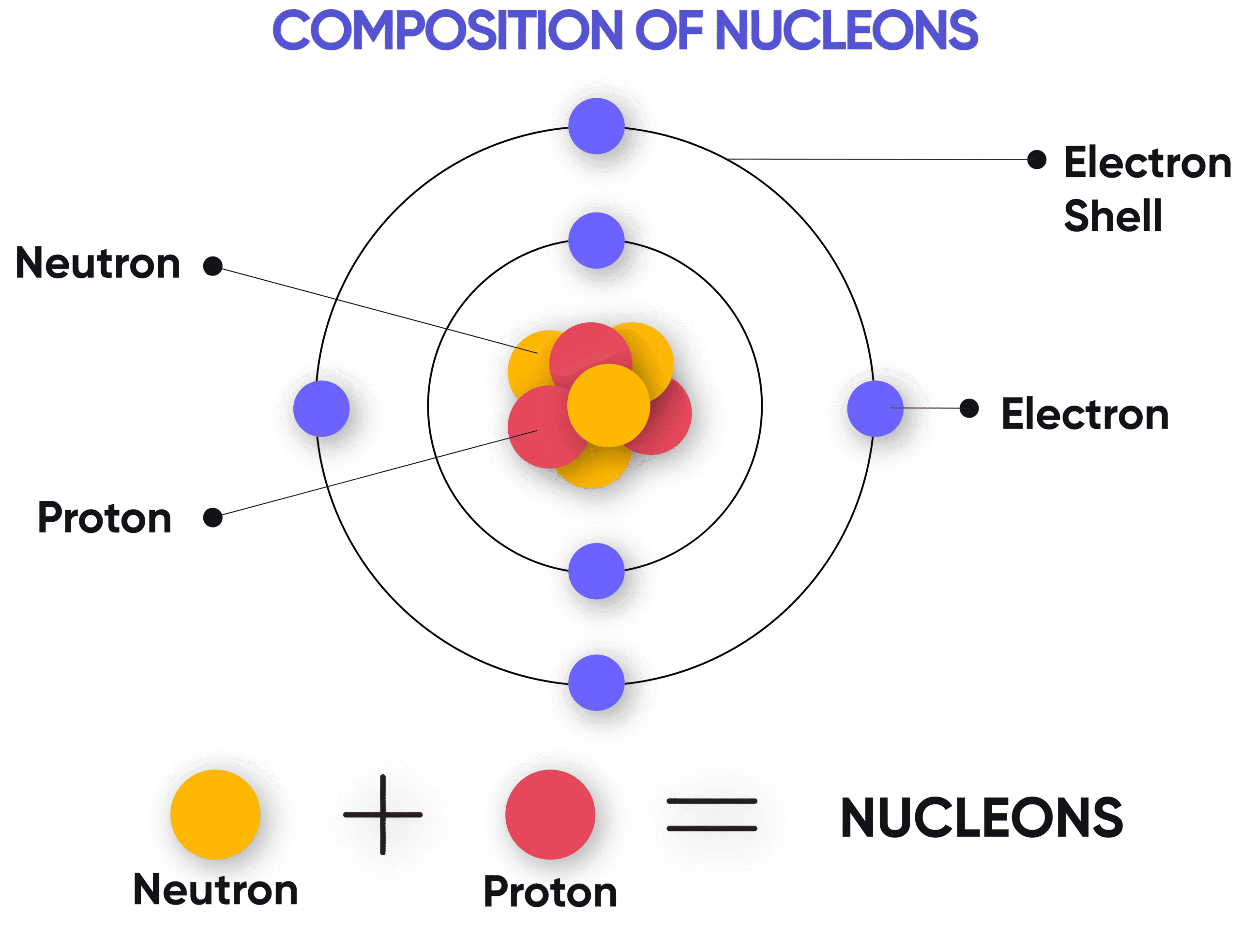
\( \mathrm{A = Z + N} \)
(where \( \mathrm{N} \) = number of neutrons)
Key Points:
- Proton number identifies the element.
- Nucleon number identifies the isotope of the element.
- Atoms with the same \( \mathrm{Z} \) but different \( \mathrm{A} \) are isotopes.
Example:
| Isotope | Symbol | Proton Number (Z) | Neutron Number (N) | Nucleon Number (A) |
|---|---|---|---|---|
| Carbon-12 | \( \mathrm{^{12}_6C} \) | 6 | 6 | 12 |
| Carbon-14 | \( \mathrm{^{14}_6C} \) | 6 | 8 | 14 |
The proton number (\( \mathrm{Z} \)) defines the type of element, while the nucleon number (\( \mathrm{A} \)) defines which isotope of that element it is.
Example
Determine the number of protons, neutrons, and electrons in a neutral atom of \( \mathrm{^{35}_{17}Cl} \).
▶️ Answer / Explanation
Proton number \( \mathrm{Z = 17} \) → 17 protons.
Nucleon number \( \mathrm{A = 35} \) → number of neutrons \( \mathrm{N = A – Z = 35 – 17 = 18.} \)
For a neutral atom, number of electrons = number of protons = 17.
Hence: 17 protons, 18 neutrons, and 17 electrons.
Understanding Isotopes
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons in their nuclei.
![]()
Explanation:
- All isotopes of an element have the same proton number (\( \mathrm{Z} \)) — so they are the same element and have identical chemical properties.
- They differ in their nucleon number (\( \mathrm{A} \)) because they have different numbers of neutrons — this affects their mass and nuclear stability.
- Some isotopes are stable (e.g., \( \mathrm{^{12}_6C} \)), while others are radioactive (e.g., \( \mathrm{^{14}_6C} \)).
Example — Isotopes of Carbon:
![]()
| Isotope | Symbol | Protons | Neutrons | Type |
|---|---|---|---|---|
| Carbon-12 | \( \mathrm{^{12}_6C} \) | 6 | 6 | Stable |
| Carbon-13 | \( \mathrm{^{13}_6C} \) | 6 | 7 | Stable |
| Carbon-14 | \( \mathrm{^{14}_6C} \) | 6 | 8 | Radioactive (β⁻ emitter) |
Isotopes are atoms of the same element (same \( \mathrm{Z} \)) with different numbers of neutrons (different \( \mathrm{A} \)). They share chemical properties but differ in nuclear and physical properties such as mass and stability.
Example
State one similarity and one difference between \( \mathrm{^{35}_{17}Cl} \) and \( \mathrm{^{37}_{17}Cl} \).
▶️ Answer / Explanation
Similarity: Both have the same number of protons (17) and electrons, so they exhibit identical chemical properties.
Difference: They have different numbers of neutrons — 18 and 20 respectively — leading to different masses and slightly different physical properties.
Nuclear Notation (\( \mathrm{^A_ZX} \))
The standard notation \( \mathrm{^A_ZX} \) represents a specific nuclide (a distinct nucleus of a particular isotope).![]()
Explanation of Terms:
- \( \mathrm{X} \): Chemical symbol of the element
- \( \mathrm{Z} \): Proton (atomic) number = number of protons
- \( \mathrm{A} \): Nucleon (mass) number = total number of protons + neutrons
- \( \mathrm{N = A – Z} \): Number of neutrons
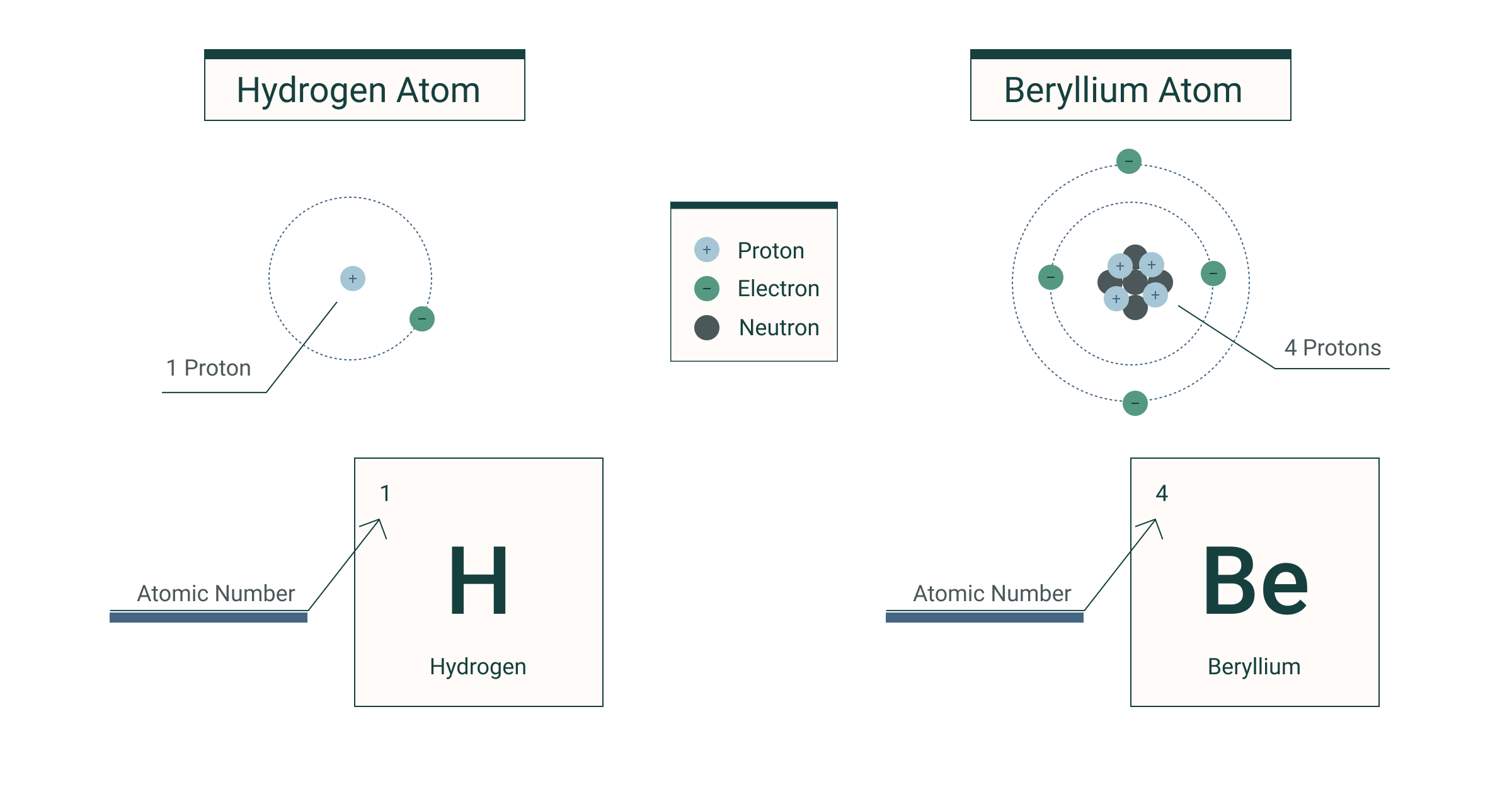
Examples:
| Nuclide | Notation | Protons (Z) | Neutrons (N) | Electrons (neutral atom) |
|---|---|---|---|---|
| Helium-4 | \( \mathrm{^4_2He} \) | 2 | 2 | 2 |
| Oxygen-16 | \( \mathrm{^{16}_8O} \) | 8 | 8 | 8 |
| Uranium-238 | \( \mathrm{^{238}_{92}U} \) | 92 | 146 | 92 |
The nuclide notation \( \mathrm{^A_ZX} \) concisely represents the atomic structure of an isotope — showing its element, total nucleons, and number of protons.
Example
Write the nuclide symbol for an atom containing 20 protons and 22 neutrons.
▶️ Answer / Explanation
Proton number \( \mathrm{Z = 20} \) → element is Calcium (\( \mathrm{Ca} \)).
Nucleon number \( \mathrm{A = 20 + 22 = 42.} \)
Nuclide notation: \( \mathrm{^{42}_{20}Ca.} \)
Conservation of Nucleon Number and Charge in Nuclear Processes
Law of Conservation in Nuclear Reactions: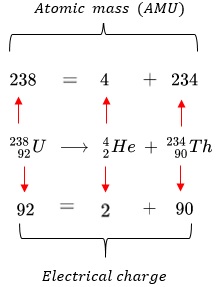
- The nucleon number (A) — the total number of protons and neutrons — is conserved in every nuclear reaction.
- The charge (proton number, Z) is also conserved — meaning the sum of proton numbers before and after a reaction remains the same.
- This applies to all nuclear processes, including radioactive decay, nuclear fission, and nuclear fusion.
![]()
In all nuclear processes, both the total number of nucleons and the total charge are conserved. Energy and momentum are also conserved overall, even though particles may transform into different types.
Example
Complete the following nuclear equation and verify conservation laws: \( \mathrm{^{210}_{84}Po \rightarrow ? + ^4_2He} \)
▶️ Answer / Explanation
Let the unknown nucleus be \( \mathrm{^A_ZX.} \)
Conserve nucleon number: \( \mathrm{210 = A + 4 \Rightarrow A = 206.} \)
Conserve proton number: \( \mathrm{84 = Z + 2 \Rightarrow Z = 82.} \)
Element with proton number 82 is Lead (\( \mathrm{Pb} \)).
Final equation: \( \mathrm{^{210}_{84}Po \rightarrow ^{206}_{82}Pb + ^4_2He} \)
Verification: Both nucleon number and charge are conserved ✅.
Composition, Mass, and Charge of α-, β-, and γ-Radiations
Radioactive decay is a spontaneous process by which unstable nuclei emit radiation in the form of particles or electromagnetic waves to become more stable. The three main types of radiation are alpha (α), beta (β), and gamma (γ).
Comparison of α, β, and γ Radiations:
| Type of Radiation | Nature / Composition | Symbol | Charge | Relative Mass | Penetrating Power | Ionising Power | Deflection by Fields |
|---|---|---|---|---|---|---|---|
| Alpha (α) | Helium nucleus (2 protons + 2 neutrons) | \( \mathrm{^4_2He} \) | \( \mathrm{+2e} \) | 4 (heavy) | Low — stopped by paper or few cm of air | High — strong ionisation | Deflected slightly towards the negative plate |
| Beta-minus (β⁻) | High-speed electron emitted from the nucleus | \( \mathrm{e^-} \) | \( \mathrm{-1e} \) | \( \approx \dfrac{1}{1836} \) | Moderate — stopped by few mm of aluminium | Moderate | Deflected strongly towards the positive plate |
| Beta-plus (β⁺) | High-speed positron (antiparticle of electron) | \( \mathrm{e^+} \) | \( \mathrm{+1e} \) | \( \approx \dfrac{1}{1836} \) | Moderate — annihilates quickly with electrons | Moderate | Deflected strongly towards the negative plate |
| Gamma (γ) | High-energy electromagnetic radiation (photon) | \( \mathrm{\gamma} \) | 0 | 0 (massless) | Very high — requires several cm of lead to stop | Very low — weakly ionising | Not deflected (no charge) |
Key Points:
- α-particles are heavy, slow, and strongly ionising but weakly penetrating.
- β-particles (electrons or positrons) are light, faster, and more penetrating but less ionising.
- γ-rays are electromagnetic waves — they have no mass or charge but are highly penetrating and weakly ionising.
Example
Arrange α, β, and γ radiations in increasing order of their penetrating power.
▶️ Answer / Explanation
Order of increasing penetration: \( \mathrm{\alpha < \beta < \gamma} \)
Alpha particles are the least penetrating because of their large mass and charge, while gamma rays, being electromagnetic, are the most penetrating.
Understanding Antiparticles — The Positron
Every particle has a corresponding antiparticle — a particle with the same mass but opposite charge (and opposite quantum numbers such as lepton or baryon number).
Key Properties of Antiparticles: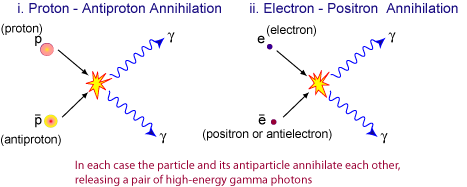
- Same mass as the corresponding particle.
- Opposite electric charge and quantum properties.
- When a particle meets its antiparticle, they annihilate — converting all mass into energy (usually γ-rays).
The Positron:
- A positron (\( \mathrm{e^+} \)) is the antiparticle of the electron.
- It has the same mass as the electron but a positive charge.
- Symbol: \( \mathrm{e^+} \) or \( \mathrm{\beta^+} \)
- Charge: \( \mathrm{+1.6 \times 10^{-19} \ C} \)
- Mass: \( \mathrm{9.11 \times 10^{-31} \ kg} \)
- Occurs in β⁺ decay processes.
Example — Electron–Positron Pair:
| Property | Electron (\( \mathrm{e^-} \)) | Positron (\( \mathrm{e^+} \)) |
|---|---|---|
| Charge | \( \mathrm{-1e} \) | \( \mathrm{+1e} \) |
| Mass | \( \mathrm{9.11 \times 10^{-31} \ kg} \) | \( \mathrm{9.11 \times 10^{-31} \ kg} \) |
| Interaction when combined | Annihilate to produce γ-ray photons | |
A positron is the antiparticle of the electron — same mass, opposite charge. When they meet, they annihilate, producing energy in the form of gamma rays.
Example
What happens when a positron meets an electron?
▶️ Answer / Explanation
When a positron (\( \mathrm{e^+} \)) and an electron (\( \mathrm{e^-} \)) collide, they annihilate each other, converting their mass into energy:
\( \mathrm{e^+ + e^- \rightarrow 2\gamma} \)
Two gamma photons are produced, conserving both energy and momentum. This process is known as pair annihilation.
Neutrinos and Antineutrinos in Beta Decay
Neutrinos and antineutrinos are fundamental leptons with extremely small mass and no electric charge. They are produced in beta decay processes to conserve energy, momentum, and lepton number.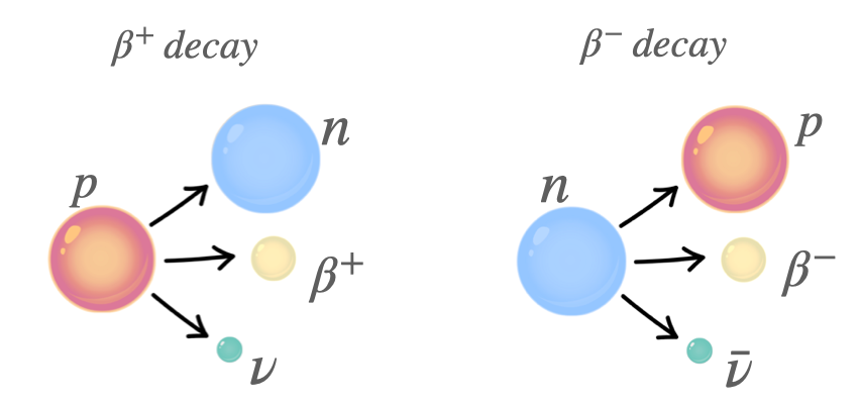
Types of Neutrinos:
- Electron neutrino (\( \mathrm{\nu_e} \))
- Electron antineutrino (\( \mathrm{\bar{\nu}_e} \))
In Beta Decays:
| Type of Decay | Equation | Lepton Emitted | Conservation Role |
|---|---|---|---|
| β⁻ decay | \( \mathrm{n \rightarrow p + e^- + \bar{\nu}_e} \) | Electron and electron antineutrino | \( \mathrm{\bar{\nu}_e} \) ensures conservation of energy and lepton number |
| β⁺ decay | \( \mathrm{p \rightarrow n + e^+ + \nu_e} \) | Positron and electron neutrino | \( \mathrm{\nu_e} \) ensures conservation of lepton number and energy |
Explanation:
- In β⁻ decay, a neutron changes into a proton and emits an electron and an antineutrino (\( \mathrm{\bar{\nu}_e} \)).
- In β⁺ decay, a proton changes into a neutron and emits a positron and a neutrino (\( \mathrm{\nu_e} \)).
- These particles carry away excess energy and conserve lepton number (electron and neutrino balance).
Electron antineutrinos (\( \mathrm{\bar{\nu}_e} \)) are produced in β⁻ decay, and electron neutrinos (\( \mathrm{\nu_e} \)) are produced in β⁺ decay. Both are fundamental, neutral leptons with extremely small mass.
Example
Identify the lepton emitted and explain its role in each of the following decays:
(a) \( \mathrm{^{14}_6C \rightarrow ^{14}_7N + e^- + ?} \)
(b) \( \mathrm{^{22}_{11}Na \rightarrow ^{22}_{10}Ne + e^+ + ?} \)
▶️ Answer / Explanation
(a) In β⁻ decay, the missing particle is an electron antineutrino (\( \mathrm{\bar{\nu}_e} \)). It carries away excess energy and ensures lepton number conservation.
(b) In β⁺ decay, the missing particle is an electron neutrino (\( \mathrm{\nu_e} \)). It balances the lepton number, since a positron (antilepton) is emitted.
Final equations:
(a) \( \mathrm{^{14}_6C \rightarrow ^{14}_7N + e^- + \bar{\nu}_e} \)
(b) \( \mathrm{^{22}_{11}Na \rightarrow ^{22}_{10}Ne + e^+ + \nu_e} \)
Discrete and Continuous Energies of α- and β-Particles
The difference in the energy distribution of α-particles and β-particles arises from whether a neutrino or antineutrino is emitted during the decay process.
(a) α-Particles — Discrete Energies
![]()
- In α-decay, a nucleus emits an α-particle (a helium nucleus \( \mathrm{^4_2He} \)).
- The decay process involves only two products — the α-particle and the daughter nucleus.
- Energy and momentum are shared only between these two particles, so each α-particle has a fixed (discrete) energy.
- Each isotope emits α-particles with characteristic discrete energies, acting like a nuclear “fingerprint.”
Example: \( \mathrm{^{238}_{92}U \rightarrow ^{234}_{90}Th + ^4_2He} \)
→ α-particle emitted with a discrete energy (specific to uranium-238 decay).
(b) β-Particles — Continuous Range of Energies
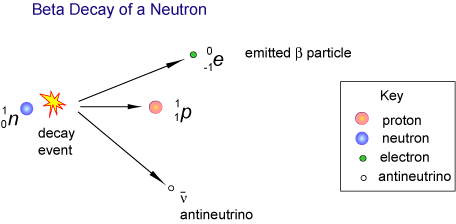
- In β-decay, a neutron transforms into a proton (β⁻ decay) or vice versa (β⁺ decay), producing three particles — a β-particle (electron or positron), a (anti)neutrino, and a daughter nucleus.
- Since three particles share the available energy, the β-particle does not always receive the same amount — leading to a continuous spectrum of energies.
- The missing energy is carried away by the (anti)neutrino, which was proposed to explain this observation.
Example: \( \mathrm{^{14}_6C \rightarrow ^{14}_7N + e^- + \bar{\nu}_e} \)
→ β⁻ particle emitted with variable kinetic energy; remaining energy goes to the antineutrino.
Comparison:
| Property | α-Particles | β-Particles |
|---|---|---|
| Particles emitted | 2 (α + daughter nucleus) | 3 (β + (anti)neutrino + daughter nucleus) |
| Energy distribution | Discrete (fixed energies) | Continuous (variable energies) |
| Reason | Energy shared between only two particles | Energy shared among three particles |
| Example | \( \mathrm{^{238}_{92}U \rightarrow ^{234}_{90}Th + ^4_2He} \) | \( \mathrm{^{14}_6C \rightarrow ^{14}_7N + e^- + \bar{\nu}_e} \) |
Example
Explain why the α-particles emitted by \( \mathrm{^{226}_{88}Ra} \) all have the same discrete kinetic energy, whereas the β⁻ particles emitted by \( \mathrm{^{14}_6C} \) have a continuous range of energies.
▶️ Answer / Explanation
For α-decay (discrete energies):
- The decay equation is \( \mathrm{^{226}_{88}Ra \rightarrow ^{222}_{86}Rn + ^4_2He.} \)
- Only two particles are produced — the α-particle and the daughter nucleus \( \mathrm{Rn.} \)
- By the law of conservation of energy and momentum, the total decay energy (Q-value) is shared only between these two bodies.
- Thus, the α-particles are emitted with a single, fixed kinetic energy (for a specific transition of the nucleus).
- If the nucleus has multiple energy levels, a few discrete α energies may appear — one for each transition.
For β⁻-decay (continuous energies):
- The decay equation is \( \mathrm{^{14}_6C \rightarrow ^{14}_7N + e^- + \bar{\nu}_e.} \)
- Three particles share the available decay energy: the β⁻ particle (electron), the antineutrino, and the daughter nucleus.
- Because the energy can be divided in infinitely many ways between the β⁻ and the \( \bar{\nu}_e \), the electron’s kinetic energy is not fixed.
- This results in a continuous energy spectrum for β⁻ particles, ranging from 0 up to a maximum value determined by the Q-value of the decay.
Conclusion:
- α-decay → 2-body system → discrete energies.
- β-decay → 3-body system → continuous energy spectrum because of neutrino emission.
Graphical Comparison:
The β-particle spectrum is continuous, whereas α-particles have discrete lines (sharp energies).
Representing α- and β-Decay Using Radioactive Decay Equations
A radioactive decay equation shows how an unstable nucleus transforms into a more stable one by emitting α, β, or γ radiation. It must obey conservation of charge and nucleon number.
(a) Alpha Decay:
![]()
- Occurs when a heavy nucleus emits an α-particle (\( \mathrm{^4_2He} \)).
- Nucleon number decreases by 4, and proton number decreases by 2.
\( \mathrm{^{238}_{92}U \rightarrow ^{234}_{90}Th + ^4_2He} \)
Verification:
- Nucleon number: \( \mathrm{238 = 234 + 4} \)
- Proton number: \( \mathrm{92 = 90 + 2} \)
(b) Beta-minus (β⁻) Decay: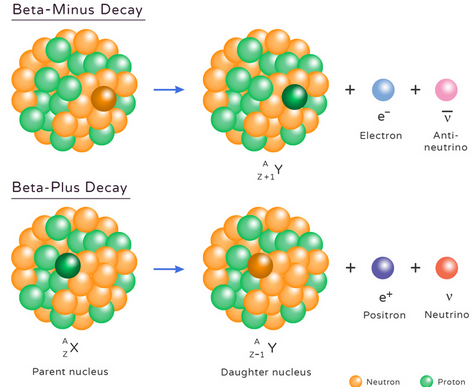
- A neutron in the nucleus converts into a proton, emitting an electron (β⁻) and an antineutrino.
- Nucleon number remains constant; proton number increases by 1.
\( \mathrm{^{14}_6C \rightarrow ^{14}_7N + e^- + \bar{\nu}_e} \)
(c) Beta-plus (β⁺) Decay:
- A proton changes into a neutron, emitting a positron (β⁺) and a neutrino.
- Nucleon number remains constant; proton number decreases by 1.
\( \mathrm{^{22}_{11}Na \rightarrow ^{22}_{10}Ne + e^+ + \nu_e} \)
Key Rules for Writing Decay Equations:
- Conserve nucleon number (A).
- Conserve charge/proton number (Z).
- Include emitted particles (α, β, γ, ν, or \( \bar{\nu} \)) explicitly.
Example
Complete the decay equation: \( \mathrm{^{210}_{84}Po \rightarrow ? + ^4_2He} \)
▶️ Answer / Explanation
Conserve nucleon number: \( \mathrm{210 = A + 4 \Rightarrow A = 206.} \)
Conserve proton number: \( \mathrm{84 = Z + 2 \Rightarrow Z = 82.} \)
Element with Z = 82 → Lead (Pb).
Equation: \( \mathrm{^{210}_{84}Po \rightarrow ^{206}_{82}Pb + ^4_2He.} \)
Example
Write the balanced nuclear equation for β⁻ decay of strontium-90.
▶️ Answer / Explanation
In β⁻ decay: proton number increases by 1, nucleon number unchanged.
\( \mathrm{^{90}_{38}Sr \rightarrow ^{90}_{39}Y + e^- + \bar{\nu}_e} \)
Yttrium-90 is the daughter nucleus.
Unified Atomic Mass Unit (u)
The unified atomic mass unit (u) is a standard unit of atomic and subatomic mass.
It is defined as:
\( \mathrm{1 \ u = \dfrac{1}{12} \ of \ the \ mass \ of \ one \ atom \ of \ ^{12}C.} \)
In SI Units:
\( \mathrm{1 \ u = 1.6605 \times 10^{-27} \ kg.} \)
Approximate Masses of Subatomic Particles:
| Particle | Symbol | Mass (u) | Mass (kg) | Charge |
|---|---|---|---|---|
| Proton | \( \mathrm{p} \) | 1.0073 | \( \mathrm{1.673 \times 10^{-27}} \) | \( \mathrm{+1e} \) |
| Neutron | \( \mathrm{n} \) | 1.0087 | \( \mathrm{1.675 \times 10^{-27}} \) | 0 |
| Electron | \( \mathrm{e^-} \) | 0.00055 | \( \mathrm{9.11 \times 10^{-31}} \) | \( \mathrm{-1e} \) |
Usage:
- Used to express atomic and nuclear masses conveniently without very small SI numbers.
- 1 u corresponds approximately to the mass of 1 nucleon (proton or neutron).
- Useful in calculating mass defect and binding energy in nuclear physics.
Example
Calculate the mass of a helium nucleus (\( \mathrm{^4_2He} \)) in kilograms.
▶️ Answer / Explanation
Given: \( \mathrm{1 \ u = 1.6605 \times 10^{-27} \ kg.} \)
Mass of helium nucleus = \( \mathrm{4 \ u = 4 \times 1.6605 \times 10^{-27} = 6.642 \times 10^{-27} \ kg.} \)
Hence: \( \mathrm{m(^4_2He) = 6.64 \times 10^{-27} \ kg.} \)
