The results of the α-particle scattering experiment & the existence and small size of the nucleus Rutherford Alpha Particle Scattering Experiment
Rutherford Alpha Particle Scattering Experiment
• Rutherford‘s alpha particle scattering experiment changed the way we think of atoms.
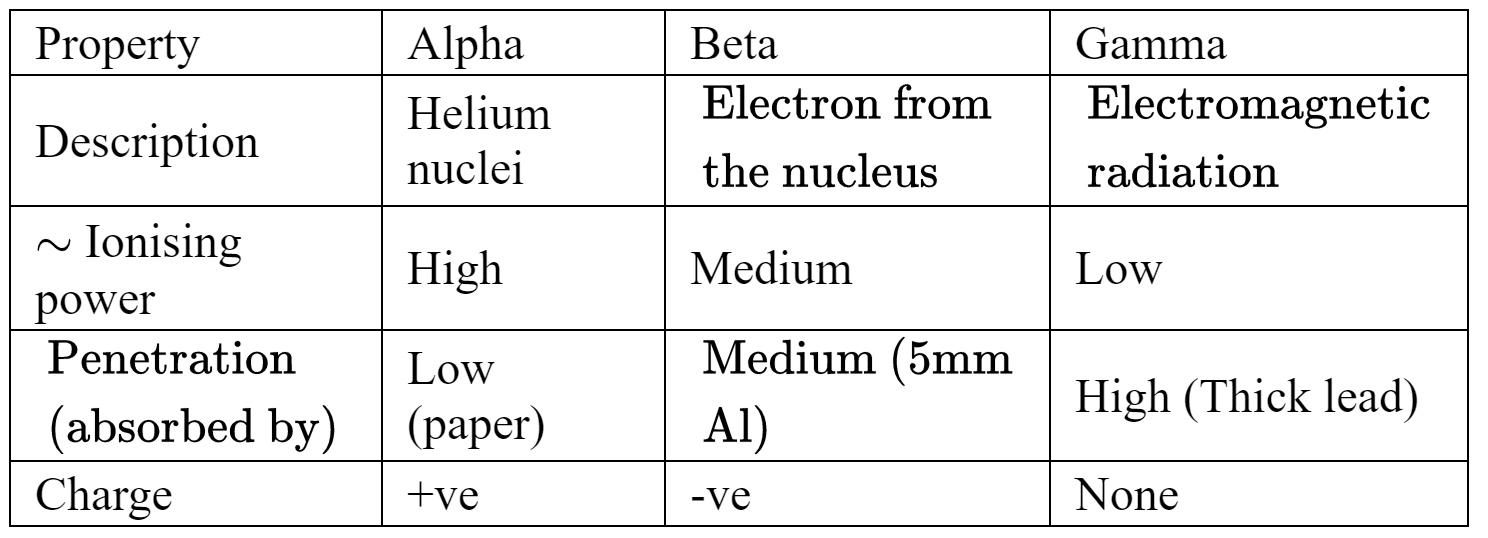
• Before the experiment the best model of the atom was known as the Thomson or “plum pudding” model. The atom was believed to consist of a positive material “pudding” with negative “plums” distributed throughout.
Rutherford Alpha Particle Scattering Experiment (continued from previous slide)

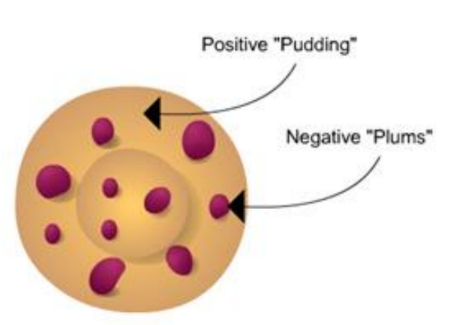
• Rutherford directed beams of alpha particles (which are the nuclei of helium atoms and hence positively charged) at thin gold foil to test this model and noted how the alpha particles scattered from the foil.
Rutherford made 3 observations:
- Most of the fast, highly charged alpha particles went whizzing straight through un-deflected. This was the expected result for all of the particles if the plum pudding model was correct.
- Some of the alpha particles were deflected back through large angles. This was not expected.
- A very small number of alpha particles were deflected backwards! This was definitely not as expected.
- To explain these results a new model of the atom was needed.
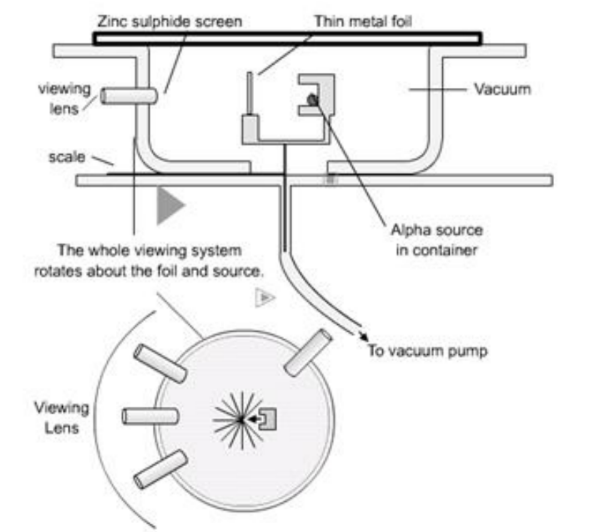
• In this model the positive material is concentrated in a small but massive (lot of mass – not size) region called the nucleus. The negative particles (electrons) must be around the outside preventing the atom from trespassing on its neighbours space to complete this model.
• The diagram in next slide will help you to understand the results of the experiment.
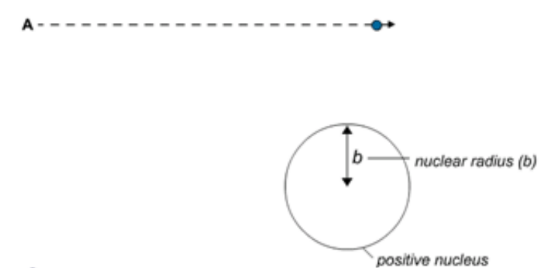
A Alpha particles this far from the nucleus experience little or no deflection as they are not close enough to the small positive nucleus.
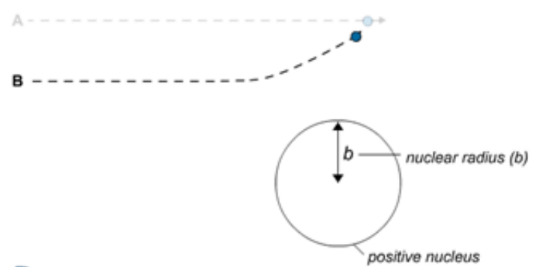
B Here, alpha particles will be slightly deflected as they are closer to the nucleus, so you will see some scattering.
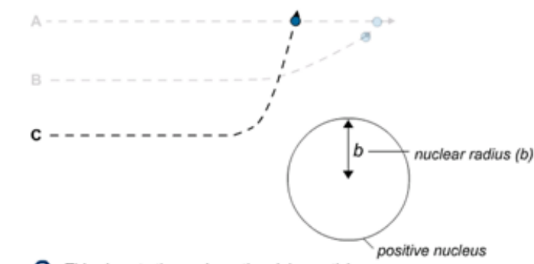
C. This close to the nucleus, the alpha particles experience a large deflection, so they are scattered through large angles.
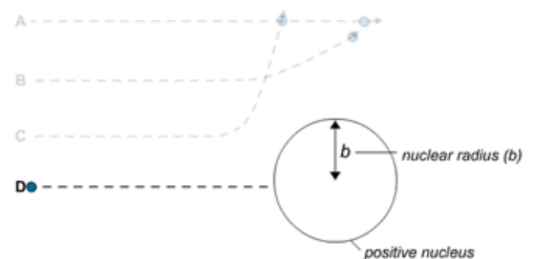
D The alpha particle has a head on collision with the nucleus so it bounces straight back
describe a simple model for the nuclear atom to include protons, neutrons and orbital electrons

Particles in the Atom
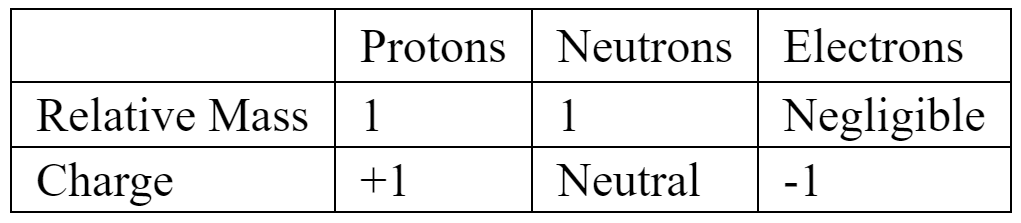
Atoms contain 3 types of particles: protons, neutrons and electrons.
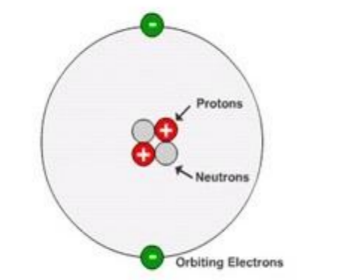
It is important to understand that the picture above is a model of the atom. It conveys an impression of what the atom is like, but is not a completely true representation.
As an example of this consider the relative sizes of the nucleus and whole atom. It can be found that a typical nuclear diameter is $1 \times 10^{-14} \mathrm{~m}$ while the typical atomic diameter is $1 \times 10^{-10} \mathrm{~m}$. Thus the nucleus is around 10,000 times smaller than the entire atom. You could build a model of an atom by placing a pea on the centre spot of a football stadium (to represent the nucleus) and then placing the electrons somewhere out in the stands. The picture above certainly does not reflect this fact accurately! Molecules are simply combinations of 1 or more atoms so are slightly larger than atoms themselves.
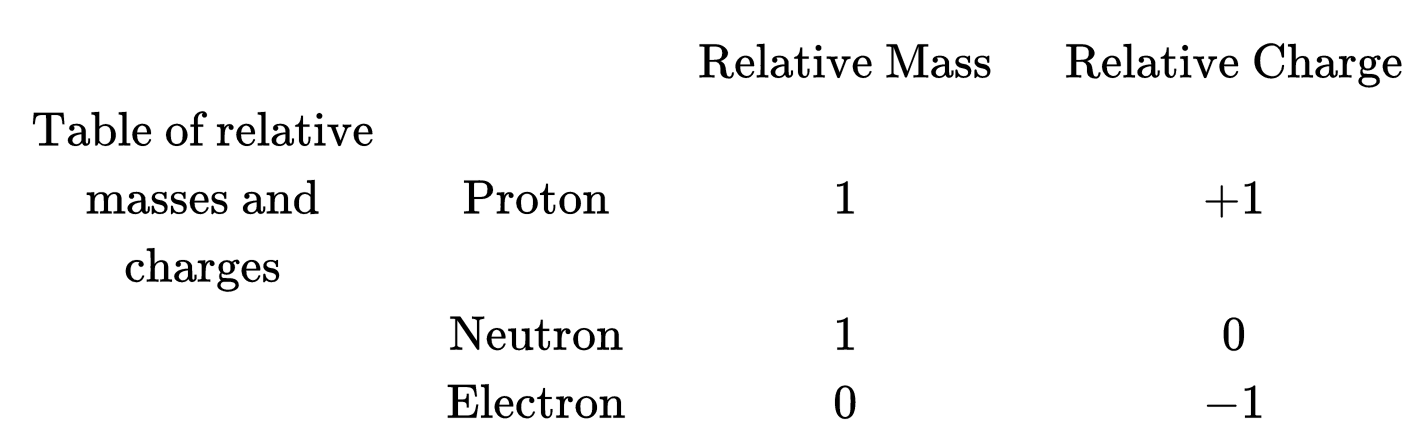
Each of these particles has a mass and a charge.

It is possible to simplify this information by looking for patterns in the numbers.
Firstly, notice that the electron and proton have equal and opposite charges. A new unit of charge called the elementary charge $\left(e=1.602 \times 10^{-19}\right.$ Coulombs) allows us to assign the values of $+1 \mathrm{e}$ and $-1 \mathrm{e}$, to the charge of these particles.
Secondly, as the mass of the proton, neutron and electron are very small a more convenient unit is the atomic mass unit $\left(\mathrm{u}=1.660 \times 10^{-27} \mathrm{~kg}\right)$. Using this new unit we can approximate the masses of the proton, neutron and electron to be $1 \mathrm{u}, 1 \mathrm{u}$ and $0 \mathrm{u}$ respectively. The relative atomic masses of the three particles can therefore simply be stated as $1,1,0$.
Many different atoms can be built using the 3 particles described above. 91 different atoms occur naturally (the chemical elements) and many more can be found in situations where energy levels are high. It is useful to have a concise way of describing these atoms.
To describe the number of particles in a given atom, we use this notation:
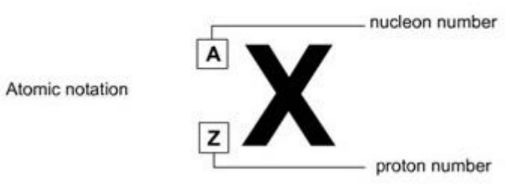
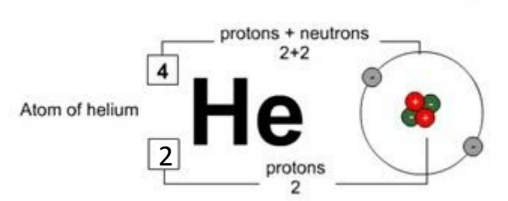
The top number (A) is called the nucleon number (as it is the number of things in the nucleus of the atom) or the mass number (as it is the mass of the atom.)
The bottom number $(Z)$ is called the proton number (as it is the number of protons) or the atomic number (as it is the number that tells you which element the atoms belongs to).
The letters give you a clue as to the name of the element. For example here is an atom of helium:
How do we know about the protons and neutrons in the nucleus?
It is an established scientific fact that the atom has a small, central nucleus containing protons and neutrons. But how did physicists gather evidence to support this view?
The Rutherford scattering experiment proved that the nucleus was small and positive but it took a different experiment to prove the existence of the protons and neutrons within. Very high-energy electrons have enough energy to actually penetrate into the nucleus itself.
Isotopes
The number of protons in an atom is crucial. It gives you the charge of the nucleus and therefore it gives you the number of electrons needed for a neutral atom. And the number of electrons governs how an atom behaves and reacts chemically with other atoms. In other words, it gives you its properties. So the number of protons makes the atom belong to a particular element. Change the number of protons and you change the element.
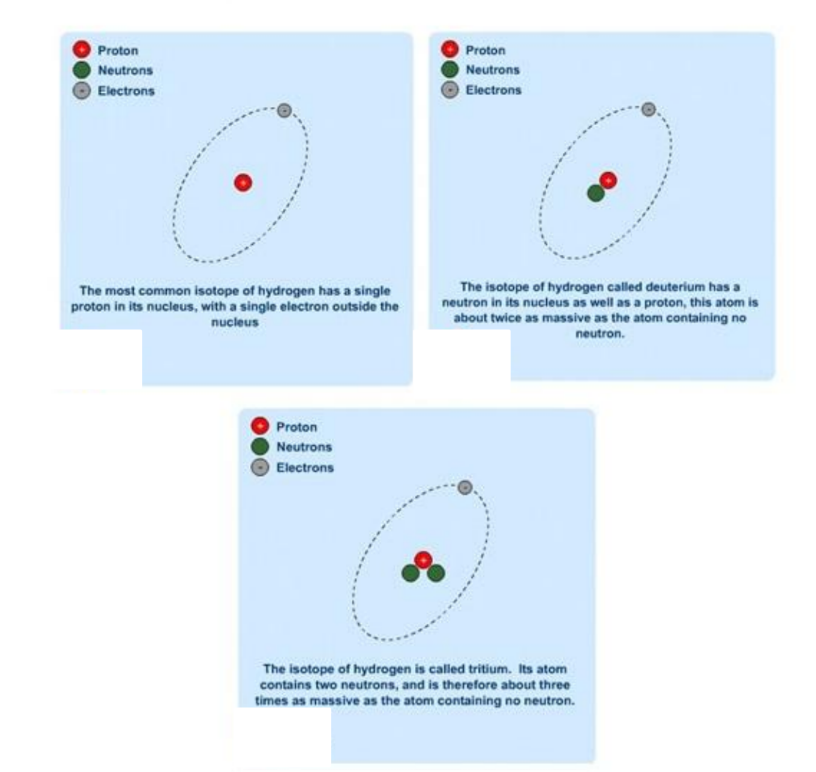
The number of neutrons in the nucleus is less crucial. You can change the number of neutrons without changing the chemical properties of the atom. So it behaves in the same way. Atoms with the same proton number but different numbers of neutrons are called isotopes.
Here are the 3 isotopes of hydrogen.
Recap…
distinguish between nucleon number and proton number
Nucleon number A: The number of nucleons (Protons and Neutrons) Proton number Z: The number of protons in the nucleus Neutron number $\mathrm{N}$ : The number of neutrons in the nucleus
show an understanding that an element can exist in various isotopic forms, each with a different number of neutrons
Nuclide: An atom with a particular number of protons and neutrons Isotope: Isotopes are nuclides that contain the same number of protons, but different numbers of neutrons.
Nucleon: Component of the nucleus = Protons and Neutrons
Decay equations
To show what happens before and after a nuclear reaction (reaction involving the nucleus of an atom) we use equations that show both the proton $(Z)$ and nucleon number $(A)$. To balance a nuclear equation (left side and right side) you have to make sure that the sum of the nucleon (top) numbers on the left hand side equals the sum of the nucleon numbers on the right hand side AND the sum of the proton numbers on both sides also balance.
For Example:
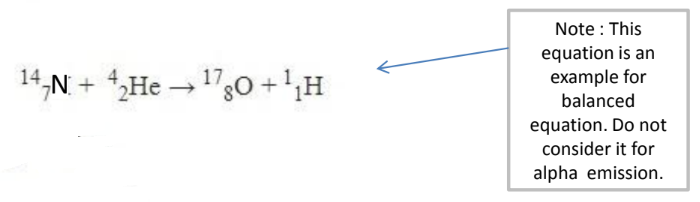
Top row $=18$ on both the left and right sides. Bottom row $=9$ on both the left and right sides.
This is a balanced equation.
Nuclear equations such as these are useful for explaining what happens in radioactive decay processes. Unstable nuclei emit alpha, beta or gamma radiation in order to become more stable. As a result of emitting this radiation the character of the nucleus remaining is changed. This is radioactive decay.
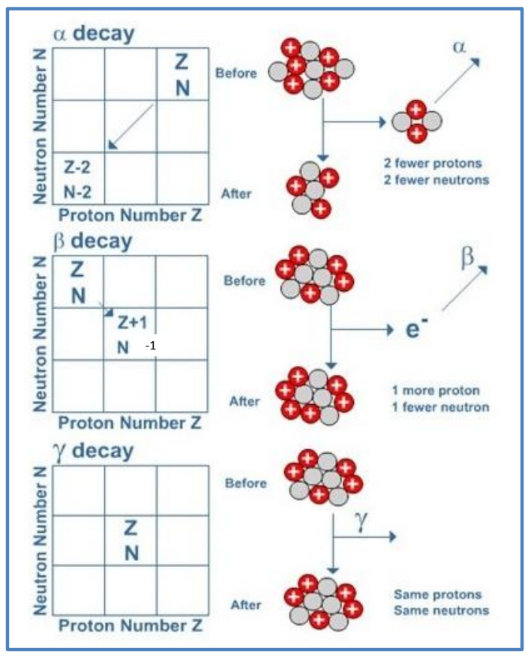
The diagrams below show what happens when a nucleus emits alpha, beta or gamma radiation.
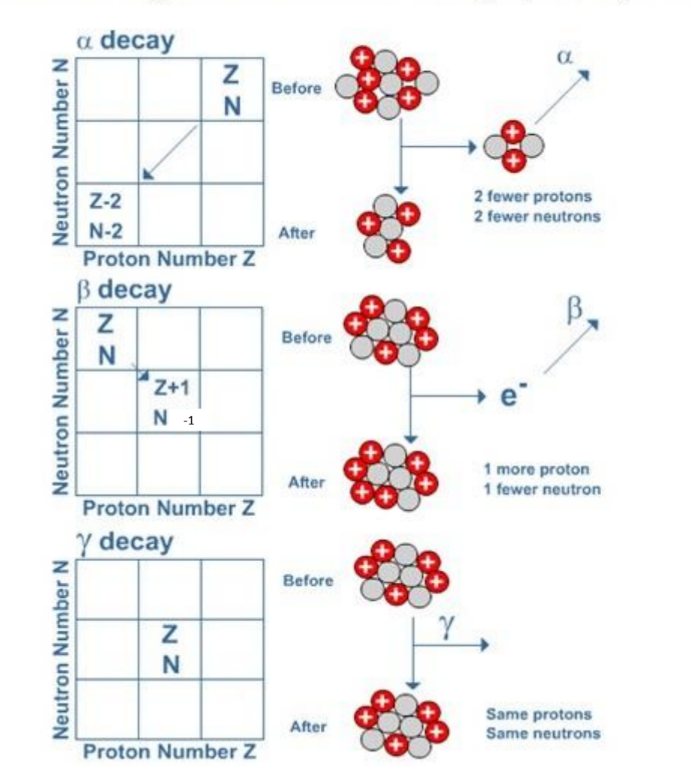
- In alpha decay 2 protons and 2 neutrons are emitted. Notice that this reduces the nucleon number by 4 and the proton number by 2 . A new element is thus formed.
- In beta decay a neutron changes into a proton (which remains in the nucleus) and an electron (which is emitted as beta radiation). The net effect is an increase in proton number by 1 , while the nucleon number stays the same. Again a new element is formed.
- When a nucleus has undergone alpha or beta decay it is often left in a highenergy (excited) state. This energy can be lost in the form of an emitted a gamma ray. Because the composition of the nucleus is unchanged no new element is formed.
Recap…
represent simple nuclear reactions by nuclear equations of the form ${ }_7^{14} \mathrm{~N}+{ }_2{ }_2 \mathrm{He}-{ }_{17}{ }_8 \mathrm{O}+{ }^1{ }_1 \mathrm{H}$
Alpha decay….
Example….
$
{ }_Z^A X \rightarrow{ }_{Z-2}^{A-4} Y+{ }_2^4 \alpha
$
$
{ }_{95}^{241} \mathrm{Am} \rightarrow{ }_{93}^{237} \mathrm{~Np}+{ }_2^4 \alpha
$
Beta decay
$
{ }_Z^A X \rightarrow{ }_{Z+1}^A Y+{ }_{-1}^0 \beta+\bar{v}
$
$
{ }_{38}^{90} \mathrm{Sr} \rightarrow{ }_{39}^{90} Y+{ }_{-1}^0 \beta+\bar{v}
$
Gamma decay
$
{ }_Z^A X \rightarrow{ }_z^A X+{ }_0^0 \gamma
$
In any nuclear reaction the following must always be true:
- The total atomic number before the reaction must be the same as the total atomic number after the reaction.
- The total atomic mass before the reaction must be the same as the total atomic mass after the reaction.
The first requirement above is the statement of the conservation of charge in nuclear reactions. The second requirement is the statement of the conservation of nucleon number.
Why are Some Atoms Radioactive?
Instability
Some atoms are unstable. They have too much energy or the wrong mix of particles in the nucleus. So to make themselves more stable, they breakdown (or decay) and get rid of some matter and/or some energy. This is called radioactive decay and isotopes of atoms that do this are called radioisotopes.
The process is spontaneous and random. You can’t do anything to speed it up or slow it down and you can’t predict when it will happen. The only reason we can do any calculations on radioisotopes is because there are huge numbers of atoms in most samples so we can use statistics to accurately predict what’s most likely to happen.
Background Radiation
A Geiger counter set up anywhere on Earth will always register a count. This is due to tiny fragments of radioactive elements present in all rocks and soil, the atmosphere and even living material. The Earth is also continuously bombarded by high-speed particles from outer space and the Sun called cosmic rays. In addition the nuclear and health industries produce small amounts of radiation each year. Collectively this radiation around us from natural and unnatural sources is called background radiation.
The chart shows the main sources of background radiation.
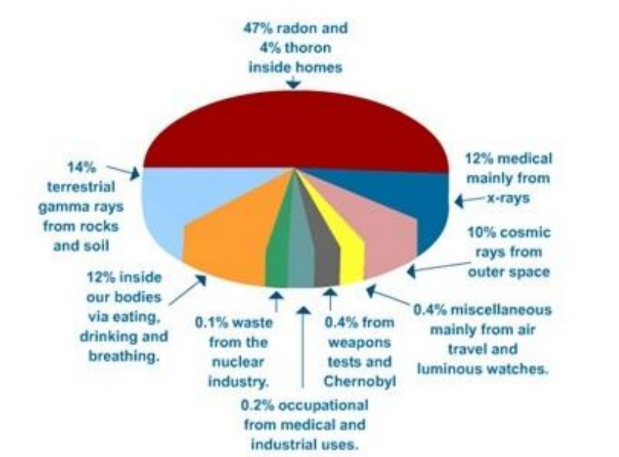
When carrying out practical work involving count-rates from radioactive sources, allowance should be made for this background radiation. This can usually be done effectively by measuring the background count in the laboratory for several minutes, and subtracting the appropriate amount from subsequent readings taken with the source.
What is ionising radiation?
Alpha, beta and gamma
Ionising radiation comes in three varieties:
$\alpha$ (alpha) particles
$\beta$ (beta) particles
$\gamma$ (gamma) rays.
All of these forms of radiation are energetic enough to pull electrons away from atoms. The atoms that have had electrons removed in this way are now charged particles, or ions, and hence the name ionising radiation.
The fact that these radiations are ionising allows them to be detected and discriminated from other forms of radiation (such as infra-red or radiowaves). Detectors such as ionisation chambers, GeigerMuller tubes and cloud chambers all rely on the ionising properties of these radiations to produce measurable effects.
Properties of alpha, beta and gamma radiation
Alpha Particles.
Alpha particles are strongly ionising but can be stopped by paper or skin. They have a strong positive charge $(+2)$ and a mass of 4 (i.e. 4 times the mass of a proton)
An alpha particle is in fact the same as a helium nucleus -2 protons and 2 neutrons.
Beta particles.
Beta particles are electrons – but they are called beta particles to identify that they came from the nucleus of the atom.
How do you get an electron from the nucleus? A neutron splits up and becomes a proton and an electron. The proton remains behind in the nucleus, the electron is emitted.
Beta particles are also strongly ionising (perhaps 1 beta particle will cause 100 ionisations).
Gamma Rays.
Gamma rays are very poor at ionising (about 1 to 1 ) but they are very difficult to stop (they are very penetrating). As they are not good ionisers, they are less dangerous to life.
They are in fact pure energy (at the shortest wavelength end of the E-M spectrum) and gamma emission accompanies most emissions of beta or alpha particles.
Recap….
show an understanding of the nature and properties of $\alpha$-, $\beta$-and $\gamma$-radiations ( $\beta+$ is not included: $\beta$ – radiation will be taken to refer to $\beta-$ )