Thermal Energy Transfer
- The conservation of energy states that energy is never created or destroyed, only transferred from one form to another
- When a thermometer is placed in a beaker of boiling water, the thermometer reading increases
- This is because the thermometer is a lot cooler than the water
- The thermometer gradually becomes hotter from the thermal energy (or heat) transferring from the water to the thermometer
- Thermal energy is defined as:
Thermal energy is transferred from a region of higher temperature to a region of lower temperature
- The energy will continue to be transferred until both the thermometer and the water are at the same temperature
- This means temperature tells us the direction of energy flow when two regions are in contact (from hotter to cooler)
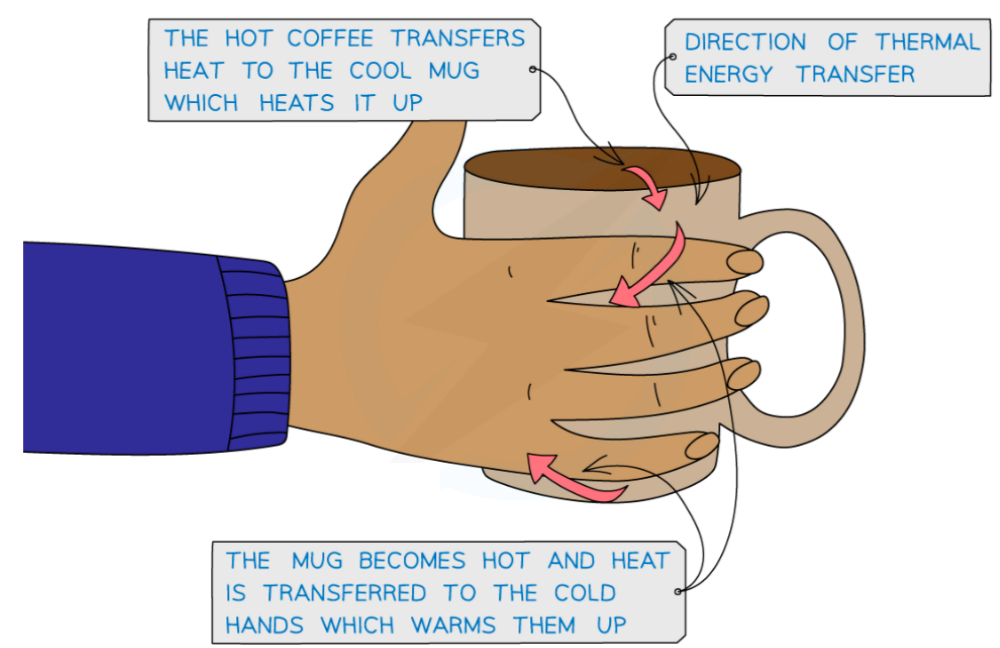
Thermal energy is transferred from the hot coffee to the mug and to the cold hands
- The mechanism by which the thermal energy is transferred is by either conduction, convection or radiation
Exam Tip
Sometimes the direction of heat transfer might seem counterintuitive to what we observe in everyday life. When ice is placed in room temperature water, it melts. This is because the water transfers heat energy to the ice (not the ice giving it’s ‘cold’ to the water).
Defining Thermal Equilibrium
- Thermal energy is always transferred from a hotter region to lower region
- Thermal equilibrium is defined as:
When two substances in physical contact with each other no longer exchange any heat energy and both reach an equal temperature - There is no longer thermal energy transfer between the regions
- The two regions need to be in contact for this to occur
- The hotter region will cool down and the cooler region will heat up until they reach the same temperature
- The final temperature when two regions are in thermal equilibrium depends on the initial temperature difference between them
- An example of this is ice in room temperature water. The ice cubes heat up from the energy transfer from the water and the water cools down due to the ice until the water’s temperature is in thermal equilibrium
