CIE AS/A Level Physics 14.2 Temperature scales Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 14.2 Temperature scales Study Notes – New Syllabus
CIE AS/A Level Physics 14.2 Temperature scales Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- understand that a physical property that varies with temperature may be used for the measurement of temperature and state examples of such properties, including the density of a liquid, volume of a gas at constant pressure, resistance of a metal, e.m.f. of a thermocouple
- understand that the scale of thermodynamic temperature does not depend on the property of any particular substanc
- convert temperatures between kelvin and degrees Celsius and recall that T / K = θ / °C + 273.15
- understand that the lowest possible temperature is zero kelvin on the thermodynamic temperature scale and that this is known as absolute zero
Physical Properties Used for Measuring Temperature
A physical property that changes in a known and reproducible way with temperature can be used to define or measure temperature.
General Principle: If a property \( X \) of a material varies with temperature \( T \), then:
\( \mathrm{X = X(T)} \)
By measuring \( X \), we can determine the temperature.
Examples of Physical Properties That Vary with Temperature:
| Physical Property | How It Varies with Temperature | Example Device |
|---|---|---|
| Density of a liquid | Decreases as temperature increases (liquid expands) | Liquid-in-glass thermometer (mercury/alcohol) |
| Volume of a gas at constant pressure | Increases linearly with temperature (Charles’ law) | Gas thermometer |
| Electrical resistance of a metal | Increases with temperature due to greater lattice vibrations | Resistance thermometer (e.g., platinum resistance thermometer) |
| e.m.f. of a thermocouple | Increases (or decreases) depending on the metal pair | Thermocouple thermometer |
Explanation of Each:
- Liquid-in-glass thermometer: The liquid expands with temperature; the length of the column is used to measure temperature.
- Gas thermometer: At constant pressure, the volume of gas is directly proportional to thermodynamic temperature.
- Resistance thermometer: Metals have higher resistance at higher temperatures.
- Thermocouple: Two different metals produce an e.m.f. proportional to the temperature difference between their junctions.
Many physical properties depend on temperature and can be used to construct a temperature scale—but each depends on the substance used.
Example
Explain why a mercury thermometer rises when placed in hot water.
▶️ Answer / Explanation
The hot water has a higher temperature than the mercury. Thermal energy is transferred to the mercury, causing it to expand. As its volume increases, the mercury rises up the glass tube. The height of the column corresponds to the temperature.
Example
Why is a platinum resistance thermometer used for precise measurements?
▶️ Answer / Explanation
Platinum has a very stable and nearly linear relationship between resistance and temperature. Small changes in temperature produce measurable changes in resistance, making it accurate and suitable for laboratory standards.
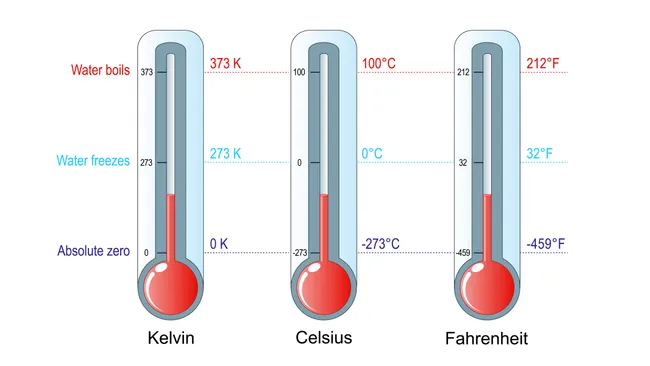
Understanding the Thermodynamic Temperature Scale
The thermodynamic temperature scale (Kelvin scale) is an absolute temperature scale based only on the laws of thermodynamics. It does not depend on the physical properties of any specific substance.
Key Features:
- Based on absolute zero — the lowest possible temperature where particles have minimum kinetic energy.
- Zero kelvin (0 K) is defined as the temperature at which a system has minimal internal energy.
- A degree kelvin is the same size as a degree Celsius, but the scale starts at absolute zero.
- The scale is independent of material properties (unlike mercury, gas, resistance thermometers).
The thermodynamic temperature scale is universal and does not rely on how any specific substance behaves with temperature.
Why this is important:
- Material properties may be nonlinear or change with conditions.
- The Kelvin scale allows scientists to compare temperatures objectively.
- It is based on the ideal gas law and the second law of thermodynamics.
Example
Why is the Kelvin scale preferred in scientific calculations?
▶️ Answer / Explanation
The Kelvin scale is absolute and substance-independent. It starts at absolute zero, where molecular kinetic energy is minimum. Equations of thermodynamics (ideal gas law, kinetic theory) work correctly only when temperature is in kelvin.
Example
Explain why the thermodynamic temperature scale does not use the expansion of mercury to define temperature.
▶️ Answer / Explanation
A property like mercury expansion depends on how that particular substance behaves, which may be nonlinear or vary with pressure and impurities. The thermodynamic scale must be universal and independent of material behavior, so it is defined using fundamental thermodynamic laws instead of material properties.
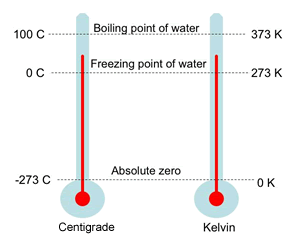
Conversion Between Kelvin and Degrees Celsius
The kelvin (K) and degree Celsius (°C) scales have equal-sized units; they differ only in their zero points.
\( \mathrm{T \ (K) = \theta \ (^{\circ}C) + 273.15} \)
\( \mathrm{\theta \ (^{\circ}C) = T \ (K) – 273.15} \)
Explanation:
- The Kelvin scale starts at absolute zero (0 K), while the Celsius scale starts at the freezing point of water (0°C).
- There is a fixed difference of 273.15 between them.
- A change of 1°C is equal to a change of 1 K.
Kelvin = Celsius + 273.15
Example
Convert \( \mathrm{20^{\circ}C} \) to kelvin.
▶️ Answer / Explanation
Using \( \mathrm{T = \theta + 273.15} \):
\( \mathrm{T = 20 + 273.15 = 293.15 \ K.} \)
So, \( \mathrm{20^{\circ}C = 293.15 \ K.} \)
Example
Convert \( \mathrm{310 \ K} \) to degrees Celsius.
▶️ Answer / Explanation
Using \( \mathrm{\theta = T – 273.15} \):
\( \mathrm{\theta = 310 – 273.15 = 36.85^{\circ}C.} \)
So, \( \mathrm{310 \ K = 36.85^{\circ}C.} \)
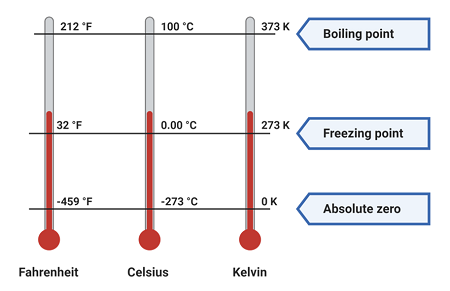
Understanding Absolute Zero
Absolute zero is the lowest possible temperature, equal to:
\( \mathrm{0 \ K = -273.15^{\circ}C} \)
Conceptual Meaning:
- At absolute zero, particles have minimum possible internal energy.
- There is no heat energy left to be removed from the system.
- Particles do not stop moving completely but have minimal vibrational energy (quantum mechanics).
- It is physically impossible to reach absolute zero exactly, but temperatures can be approached very closely in experiments.
Thermodynamic Temperature Scale:
- The Kelvin scale begins at absolute zero.
- No temperature lower than 0 K is possible.
- Kelvin temperatures measure absolute molecular energy.
Absolute zero is the point where a system has the least possible thermal energy. This is why the Kelvin scale cannot have negative values.
Example
Explain why temperatures cannot be below 0 K.
▶️ Answer / Explanation
The Kelvin scale measures absolute molecular energy. At 0 K, particles have minimum internal energy. A temperature below 0 K would imply less than zero thermal energy, which is impossible. Hence, 0 K is the lower limit.
Example
If a gas thermometer reading approaches 0 K as gas pressure approaches zero, what does this imply?
▶️ Answer / Explanation
As temperature decreases, particle motion reduces, lowering pressure. When pressure approaches zero, the gas particles have minimum energy. This corresponds to absolute zero. It shows that 0 K represents the lowest thermal energy state.
