CIE AS/A Level Physics 14.3 Specific heat capacity and specific latent heat Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 14.3 Specific heat capacity and specific latent heat Study Notes – New Syllabus
CIE AS/A Level Physics 14.3 Specific heat capacity and specific latent heat Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- define and use specific heat capacity
- define and use specific latent heat and distinguish between specific latent heat of fusion and specific latent heat of vaporisation
Specific Heat Capacity
The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1 kg of the substance by 1 K (or 1°C).
\( \mathrm{Q = mc\Delta T} \)
Where:
- \( \mathrm{Q} \) = thermal energy transferred (J)
- \( \mathrm{m} \) = mass of substance (kg)
- \( \mathrm{c} \) = specific heat capacity (J kg\(^{-1}\) K\(^{-1}\))
- \( \mathrm{\Delta T} \) = temperature change (K or °C)
Specific heat capacity measures how much energy a material needs to change its temperature. Substances with high specific heat capacity (like water) warm up and cool down slowly.
| Quantity | Meaning |
|---|---|
| Specific heat capacity (c) | Energy needed to raise 1 kg by 1°C or 1 K |
| Formula | \( \mathrm{Q = mc\Delta T} \) |
| Units of c | J kg\(^{-1}\) K\(^{-1}\) |
Example
How much energy is needed to raise the temperature of 1 kg of water by 1°C? (Take \( \mathrm{c = 4200 \ J \ kg^{-1} \ K^{-1}} \)).
▶️ Answer / Explanation
Using \( \mathrm{Q = mc\Delta T} \):
\( \mathrm{Q = 1 \times 4200 \times 1 = 4200 \ J} \)
So, 4200 J of energy is required.
Example
A 0.5 kg block of metal is heated and its temperature rises from 20°C to 50°C. If \( \mathrm{c = 900 \ J \ kg^{-1} \ K^{-1}} \), find the thermal energy transferred.
▶️ Answer / Explanation
Temperature change:
\( \mathrm{\Delta T = 50 – 20 = 30°C} \)
Using \( \mathrm{Q = mc\Delta T} \):
\( \mathrm{Q = 0.5 \times 900 \times 30 = 13\,500 \ J} \)
The energy transferred is 13,500 J.
Example
A 2 kg copper block ( \( \mathrm{c = 390 \ J \ kg^{-1} \ K^{-1}} \) ) is supplied with 20 kJ of heat energy. If the block initially is at 25°C, calculate its final temperature. (Assume no heat losses.)
▶️ Answer / Explanation
Given:
- \( \mathrm{Q = 20\,000 \ J} \)
- \( \mathrm{m = 2 \ kg} \)
- \( \mathrm{c = 390 \ J \ kg^{-1} \ K^{-1}} \)
Using \( \mathrm{Q = mc\Delta T} \):
\( \mathrm{\Delta T = \dfrac{Q}{mc} = \dfrac{20\,000}{2 \times 390}} \)
\( \mathrm{\Delta T = \dfrac{20\,000}{780} \approx 25.64^\circ C} \)
Final temperature:
\( \mathrm{T_{final} = 25 + 25.64 = 50.64^\circ C} \)
The final temperature is approximately 50.6°C.
Specific Latent Heat
The specific latent heat of a substance is the amount of thermal energy required to change the state of 1 kg of the substance.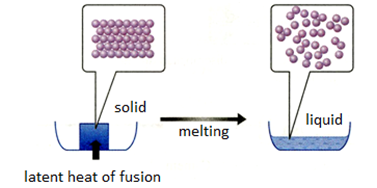
\( \mathrm{Q = mL} \)
Where:
- \( \mathrm{Q} \) = heat energy absorbed or released (J)
- \( \mathrm{m} \) = mass (kg)
- \( \mathrm{L} \) = specific latent heat (J kg\(^{-1}\))
Key Idea:
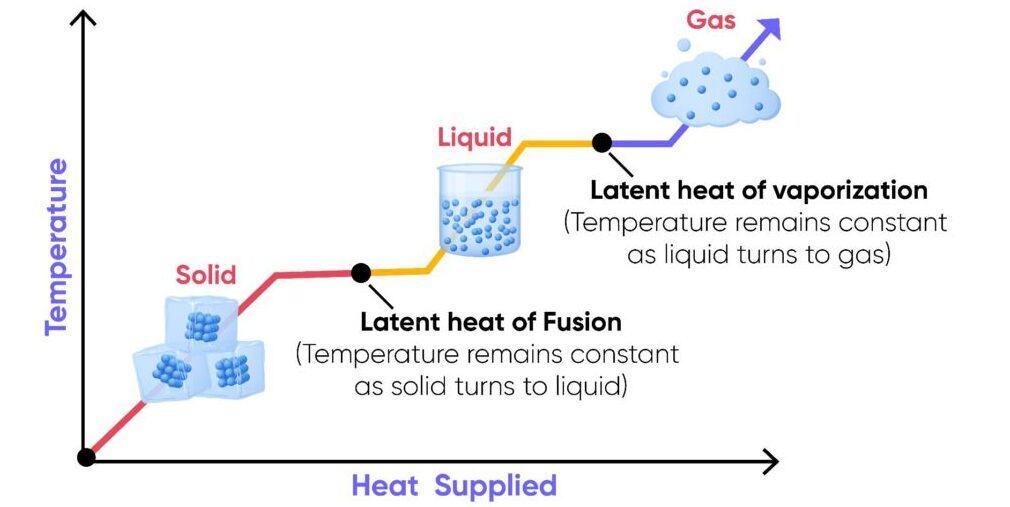
During melting, boiling, freezing, or condensation, temperature stays constant, but energy is still absorbed or released to change the state.
Types of Specific Latent Heat
| Type | Symbol | Meaning | Example Process |
|---|---|---|---|
| Specific Latent Heat of Fusion | \( \mathrm{L_f} \) | Energy needed to change 1 kg of a substance between solid ↔ liquid | Melting ice, freezing water |
| Specific Latent Heat of Vaporisation | \( \mathrm{L_v} \) | Energy needed to change 1 kg of a substance between liquid ↔ gas | Boiling water, condensing steam |
Distinction:
- \( \mathrm{L_v} \) is usually much larger than \( \mathrm{L_f} \) because converting a liquid to gas requires far more energy to overcome intermolecular forces.
- Both processes occur at constant temperature.
| Quantity | Meaning |
|---|---|
| Specific latent heat (L) | Energy needed for state change per kg, at constant temperature |
| Fusion (\(L_f\)) | Solid ↔ Liquid |
| Vaporisation (\(L_v\)) | Liquid ↔ Gas |
| Formula | \( \mathrm{Q = mL} \) |
Example
How much energy is needed to melt 0.2 kg of ice at 0°C? Take \( \mathrm{L_f = 3.34 \times 10^5 \ J \ kg^{-1}} \).
▶️ Answer / Explanation
Using \( \mathrm{Q = mL_f} \):
\( \mathrm{Q = 0.2 \times 3.34 \times 10^5 = 6.68 \times 10^4 \ J} \)
So, \( 6.68 \times 10^4 \) J of energy is needed.
Example
How much energy is needed to completely boil 0.5 kg of water at 100°C? Take \( \mathrm{L_v = 2.26 \times 10^6 \ J \ kg^{-1}} \).
▶️ Answer / Explanation
Using \( \mathrm{Q = mL_v} \):
\( \mathrm{Q = 0.5 \times 2.26\times 10^6 = 1.13\times 10^6 \ J} \)
The energy required is \( 1.13 \times 10^6 \) J.
Example
A 1.5 kg block of ice at 0°C is melted completely and then heated to 100°C, after which all the water is boiled to steam. Given:
- \( \mathrm{L_f = 3.34 \times 10^5 \ J\,kg^{-1}} \)
- \( \mathrm{L_v = 2.26 \times 10^6 \ J\,kg^{-1}} \)
- \( \mathrm{c_{water} = 4200 \ J\,kg^{-1}\,K^{-1}} \)
Find the total energy required.
▶️ Answer / Explanation
Step 1: Melt the ice
\( \mathrm{Q_1 = mL_f = 1.5 \times 3.34\times 10^5 = 5.01\times 10^5 \ J} \)
Step 2: Heat water from 0°C to 100°C
\( \mathrm{Q_2 = mc\Delta T = 1.5 \times 4200 \times 100 = 6.3 \times 10^5 \ J} \)
Step 3: Boil the water to steam
\( \mathrm{Q_3 = mL_v = 1.5 \times 2.26\times 10^6 = 3.39\times 10^6 \ J} \)
Total energy
\( \mathrm{Q_{total} = Q_1 + Q_2 + Q_3} \)
\( \mathrm{Q_{total} = (5.01 + 6.3 + 33.9)\times 10^5} \)
\( \mathrm{Q_{total} = 4.52\times 10^6 \ J} \)
The total energy required is \( 4.52 \times 10^6 \) J.
