CIE AS/A Level Physics 15.2 Equation of state Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 15.2 Equation of state Study Notes – New Syllabus
CIE AS/A Level Physics 15.2 Equation of state Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- understand that a gas obeying pV ∝ T, where T is the thermodynamic temperature, is known as an ideal gas
- recall and use the equation of state for an ideal gas expressed as pV = nRT, where n = amount of substance (number of moles) and as pV = NkT, where N = number of molecules
- recall that the Boltzmann constant k is given by k = R / NA
Ideal Gas and the Relationship \( \mathrm{pV \propto T} \)
A gas that obeys the relationship \( \mathrm{pV \propto T} \) (where \( \mathrm{T} \) is the thermodynamic temperature in kelvin) is called an ideal gas.
Mathematical Form:
\( \mathrm{\frac{pV}{T} = \text{constant}} \)
Meaning:
For a fixed mass of gas, if the temperature increases, either pressure or volume (or both) must increase to maintain the proportionality. If temperature decreases, pressure or volume decrease accordingly.
The Ideal Gas Law:
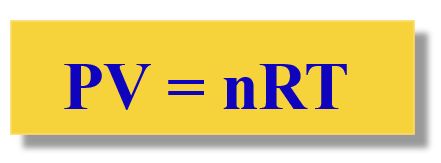
- \( \mathrm{p} \) = pressure (Pa)
- \( \mathrm{V} \) = volume (m³)
- \( \mathrm{n} \) = amount of substance (mol)
- \( \mathrm{R} \) = universal gas constant \( \mathrm{8.31\ J\,mol^{-1}\,K^{-1}} \)
- \( \mathrm{T} \) = temperature (K)
Characteristics of an Ideal Gas
- The gas particles have negligible volume compared to the container.
- There are no intermolecular forces.
- Collisions between particles and walls are perfectly elastic.
- Particles are in constant, random motion.
- The model becomes accurate at low pressures and high temperatures.
A gas is considered ideal if the ratio \( \mathrm{\dfrac{pV}{T}} \) remains constant for a fixed amount of gas.
Example
A fixed mass of gas has pressure \( \mathrm{p} \) and volume \( \mathrm{V} \) at temperature \( \mathrm{300\ K} \). If the temperature is doubled to \( \mathrm{600\ K} \) at constant volume, what happens to the pressure?
▶️ Answer / Explanation
Using \( \mathrm{p \propto T} \) at constant volume:
\( \mathrm{\frac{p_2}{p_1} = \frac{T_2}{T_1} = \frac{600}{300} = 2} \)
The pressure doubles.
Example
A fixed mass of ideal gas has an initial state: \( \mathrm{p_1 = 1.0\times 10^5\ Pa} \), \( \mathrm{V_1 = 0.020\ m^3} \), \( \mathrm{T_1 = 300\ K} \). The temperature is increased to \( \mathrm{450\ K} \), and the pressure becomes \( \mathrm{1.5\times 10^5\ Pa} \). Find the new volume \( \mathrm{V_2} \).
▶️ Answer / Explanation
Using the ideal gas relationship:
\( \mathrm{\frac{p_1 V_1}{T_1} = \frac{p_2 V_2}{T_2}} \)
Substitute values:
\( \mathrm{\frac{(1.0\times 10^5)(0.020)}{300} = \frac{(1.5\times 10^5)(V_2)}{450}} \)
Left side:
\( \mathrm{\frac{2000}{300} = 6.67} \)
Right side:
\( \mathrm{6.67 = \frac{1.5\times 10^5 \, V_2}{450}} \)
So,
\( \mathrm{V_2 = \frac{6.67 \times 450}{1.5\times 10^5}} \)
\( \mathrm{V_2 = 0.020\ m^3} \)
The volume remains the same.
Example
3 moles of an ideal gas are kept at a pressure of \( \mathrm{2.5\times 10^5\ Pa} \) and temperature \( \mathrm{400\ K} \). Calculate the volume of the gas using the ideal gas law.
▶️ Answer / Explanation
Using the ideal gas law:
\( \mathrm{pV = nRT} \)
Rearrange:
\( \mathrm{V = \frac{nRT}{p}} \)
Substitute:
\( \mathrm{V = \frac{3 \times 8.31 \times 400}{2.5\times 10^5}} \)
Calculate numerator:
\( \mathrm{3 \times 8.31 \times 400 = 9\,972} \)
Then:
\( \mathrm{V = \frac{9972}{2.5\times 10^5} = 3.99\times 10^{-2}\ m^3} \)
The volume is \( \mathrm{4.0\times 10^{-2}\ m^3} \).
Equation of State for an Ideal Gas
The behaviour of an ideal gas can be described using two equivalent forms of the equation of state:
- Molar form: \( \mathrm{pV = nRT} \)
- Molecular form: \( \mathrm{pV = NkT} \)
Molar Form — \( \mathrm{pV = nRT} \)
- \( \mathrm{p} \) = pressure (Pa)
- \( \mathrm{V} \) = volume (m³)
- \( \mathrm{n} \) = amount of substance (mol)
- \( \mathrm{R} \) = molar gas constant \( \mathrm{8.31\ J\,mol^{-1}\,K^{-1}} \)
- \( \mathrm{T} \) = thermodynamic temperature (K)
Molecular Form — \( \mathrm{pV = NkT} \)
- \( \mathrm{N} \) = number of molecules
- \( \mathrm{k} \) = Boltzmann constant \( \mathrm{k = 1.38\times 10^{-23}\ J\,K^{-1}} \)
- All other symbols have the same meaning as before.
The two forms are connected by the relationship: \( \mathrm{N = nN_A} \) and \( \mathrm{R = kN_A} \).
Both equations describe the state of an ideal gas using pressure, volume, and temperature.
Example
3 moles of an ideal gas are held in a container of volume \( \mathrm{0.050\ m^3} \) at a temperature of \( \mathrm{400\ K} \). Calculate the pressure using \( \mathrm{pV = nRT} \).
▶️ Answer / Explanation
Using the equation:
\( \mathrm{p = \frac{nRT}{V}} \)
Substitute values:
\( \mathrm{p = \frac{3 \times 8.31 \times 400}{0.050}} \)
Calculate numerator:
\( \mathrm{3 \times 8.31 \times 400 = 9\,972} \)
Then:
\( \mathrm{p = \frac{9\,972}{0.050} = 1.9944\times 10^5\ Pa} \)
The pressure is \( \mathrm{2.0\times 10^5\ Pa} \).
Example
An ideal gas has \( \mathrm{N = 5.0\times 10^{23}} \) molecules in a volume of \( \mathrm{0.040\ m^3} \) at a temperature of \( \mathrm{350\ K} \). Calculate the pressure using \( \mathrm{pV = NkT} \).
▶️ Answer / Explanation
Using the molecular form:
\( \mathrm{p = \frac{NkT}{V}} \)
Substitute values:
\( \mathrm{p = \frac{(5.0\times 10^{23})(1.38\times 10^{-23})(350)}{0.040}} \)
Compute inside the numerator:
\( \mathrm{(5.0\times 10^{23})(1.38\times 10^{-23}) = 6.9} \)
\( \mathrm{6.9 \times 350 = 2415} \)
Now divide by volume:
\( \mathrm{p = \frac{2415}{0.040} = 6.0375\times 10^4\ Pa} \)
The pressure is \( \mathrm{6.0\times 10^4\ Pa} \).
Example
A sealed rigid tank contains an ideal gas. Initially: \( \mathrm{p_1 = 1.2\times 10^5\ Pa} \), \( \mathrm{T_1 = 300\ K} \). The tank contains \( \mathrm{N = 3.0\times 10^{24}} \) molecules. The gas is heated to \( \mathrm{T_2 = 450\ K} \). Calculate the final pressure using the molecular equation of state.
▶️ Answer / Explanation
Since the container is rigid, volume is constant.
Using the proportionality: \( \mathrm{\frac{p}{T} = \text{constant}} \) for fixed \( \mathrm{N} \) and \( \mathrm{V} \)
\( \mathrm{\frac{p_2}{p_1} = \frac{T_2}{T_1}} \)
Substitute values:
\( \mathrm{\frac{p_2}{1.2\times 10^5} = \frac{450}{300}} \)
Compute ratio:
\( \mathrm{\frac{450}{300} = 1.5} \)
So:
\( \mathrm{p_2 = 1.5 \times 1.2\times 10^5 = 1.8\times 10^5\ Pa} \)
The final pressure is \( \mathrm{1.8\times 10^5\ Pa} \).
The Boltzmann Constant
The Boltzmann constant \( \mathrm{k} \) links the behaviour of a single particle to the macroscopic gas constant \( \mathrm{R} \). It is defined by the relationship:
\( \mathrm{k = \frac{R}{N_A}} \)
- \( \mathrm{R = 8.31\ J\,mol^{-1}\,K^{-1}} \) (molar gas constant)
- \( \mathrm{N_A = 6.022\times 10^{23}\ mol^{-1}} \) (Avogadro constant)
Meaning:
Boltzmann constant \( \mathrm{k} \) is the gas constant per particle instead of per mole.
It allows the ideal gas law to be written in molecular form:
\( \mathrm{pV = NkT} \)
where \( \mathrm{N} \) is the number of molecules.
Typical value:
\( \mathrm{k = 1.38\times 10^{-23}\ J\,K^{-1}} \)
Example
Calculate the value of the Boltzmann constant using \( \mathrm{R = 8.31\ J\,mol^{-1}\,K^{-1}} \) and \( \mathrm{N_A = 6.022\times 10^{23}\ mol^{-1}} \).
▶️ Answer / Explanation
\( \mathrm{k = \frac{R}{N_A} = \frac{8.31}{6.022\times 10^{23}}} \)
\( \mathrm{k = 1.38\times 10^{-23}\ J\,K^{-1}} \)
This matches the known value of \( \mathrm{k} \).
Example
An ideal gas has \( \mathrm{N = 3.0\times 10^{24}} \) molecules at a temperature of \( \mathrm{350\ K} \). Using the Boltzmann constant, calculate the product \( \mathrm{NkT} \).
▶️ Answer / Explanation
Use:
\( \mathrm{NkT = (3.0\times10^{24})(1.38\times10^{-23})(350)} \)
Step 1: Multiply the powers of ten:
\( \mathrm{3.0\times10^{24} \times 1.38\times10^{-23} = 4.14\times10^1} \)
Step 2: Multiply by 350:
\( \mathrm{41.4 \times 350 = 14\,490} \)
Thus, \( \mathrm{NkT = 1.45\times 10^4\ J} \).
Example
Show that the two equations of state \( \mathrm{pV = nRT} \) and \( \mathrm{pV = NkT} \) are consistent by substituting \( \mathrm{k = R/N_A} \) and \( \mathrm{N = nN_A} \).
▶️ Answer / Explanation
Start with the molecular form:
\( \mathrm{pV = NkT} \)
Substitute \( \mathrm{k = \frac{R}{N_A}} \) and \( \mathrm{N = nN_A} \):
\( \mathrm{pV = (nN_A)\left(\frac{R}{N_A}\right)T} \)
The \( \mathrm{N_A} \) terms cancel:
\( \mathrm{pV = nRT} \)
This proves the two equations are equivalent.
