CIE AS/A Level Physics 16.2 The first law of thermodynamics Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 16.2 The first law of thermodynamics Study Notes – New Syllabus
CIE AS/A Level Physics 16.2 The first law of thermodynamics Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- recall and use W = p∆V for the work done when the volume of a gas changes at constant pressure and understand the difference between the work done by the gas and the work done on the gas
- recall and use the first law of thermodynamics ∆U = q + W expressed in terms of the increase in internal energy, the heating of the system (energy transferred to the system by heating) and the work done on the system
Work Done by a Gas at Constant Pressure
When the volume of a gas changes at constant pressure, the work done is given by:
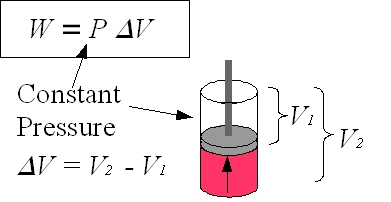
\( \mathrm{W = p \Delta V} \)
- \( \mathrm{W} \) = work done (J)
- \( \mathrm{p} \) = constant external pressure (Pa)
- \( \mathrm{\Delta V = V_{final} – V_{initial}} \) (m³)
Understanding Work Done:
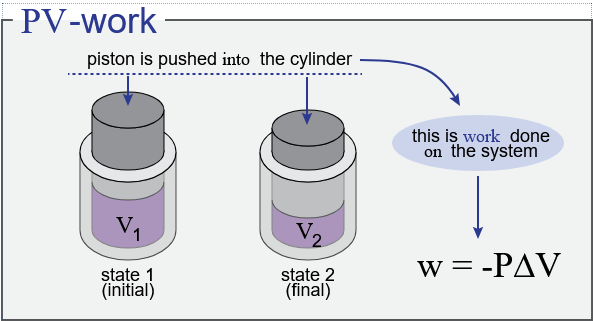
- If the gas expands (\( \mathrm{\Delta V > 0} \)), it does work on the surroundings.
- If the gas is compressed (\( \mathrm{\Delta V < 0} \)), work is done on the gas by the surroundings.
Sign Convention:
- Work done by the gas: \( \mathrm{W > 0} \)
- Work done on the gas: \( \mathrm{W < 0} \)
Physical Meaning:
When a gas expands, it pushes back the surroundings → energy is transferred from the gas → internal energy may decrease. When a gas is compressed, energy is supplied to the gas → internal energy increases.
Example
A gas expands from \( \mathrm{0.010\ m^3} \) to \( \mathrm{0.015\ m^3} \) at a constant pressure of \( \mathrm{1.2\times 10^5\ Pa} \). Calculate the work done by the gas.
▶️ Answer / Explanation
Find change in volume:
\( \mathrm{\Delta V = 0.015 – 0.010 = 0.005\ m^3} \)
Use \( \mathrm{W = p\Delta V} \):
\( \mathrm{W = (1.2\times 10^5)(0.005)} \)
\( \mathrm{W = 600\ J} \)
The gas does 600 J of work on the surroundings.
Example
A gas is compressed from \( \mathrm{0.040\ m^3} \) to \( \mathrm{0.025\ m^3} \) at a pressure of \( \mathrm{9.0\times 10^4\ Pa} \). Calculate the work done on the gas.
▶️ Answer / Explanation
Change in volume:
\( \mathrm{\Delta V = 0.025 – 0.040 = -0.015\ m^3} \)
Use \( \mathrm{W = p\Delta V} \):
\( \mathrm{W = (9.0\times 10^4)(-0.015)} \)
\( \mathrm{W = -1350\ J} \)
The negative sign means 1350 J of work is done on the gas.
Example
A piston contains gas at constant pressure \( \mathrm{2.0\times 10^5\ Pa} \). The gas absorbs 3000 J of heat energy and does 1200 J of work on the surroundings. Find the change in volume of the gas and state whether the internal energy increases or decreases.
▶️ Answer / Explanation
Use \( \mathrm{W = p\Delta V} \):
\( \mathrm{\Delta V = \frac{W}{p}} \)
\( \mathrm{\Delta V = \frac{1200}{2.0\times 10^5} = 6.0\times 10^{-3}\ m^3} \)
Change in volume = \( \mathrm{6.0\times 10^{-3}\ m^3} \) (expansion)
Internal energy:
The gas received 3000 J of heat but used 1200 J to do work.
Increase in internal energy \( \mathrm{= 3000 – 1200 = 1800\ J} \)
The internal energy of the gas increases by 1800 J.
The First Law of Thermodynamics
The first law of thermodynamics relates changes in internal energy to heating and work. It is expressed as:
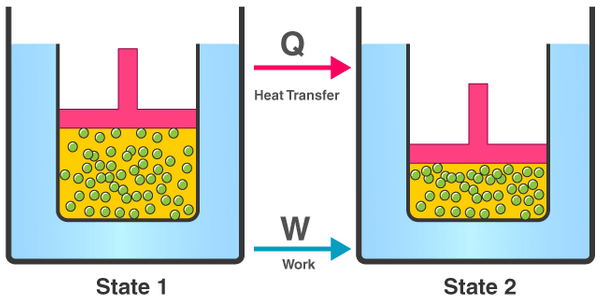
\( \mathrm{\Delta U = q + W} \)
- \( \mathrm{\Delta U} \) = change in internal energy of the system (J)
- \( \mathrm{q} \) = energy transferred to the system by heating (J)
- \( \mathrm{W} \) = work done on the system (J)
Interpretation:
Internal energy increases when heat is added to the system or when work is done on the system. Internal energy decreases when the system loses heat or does work on its surroundings.
Sign Convention (A Level Standard):
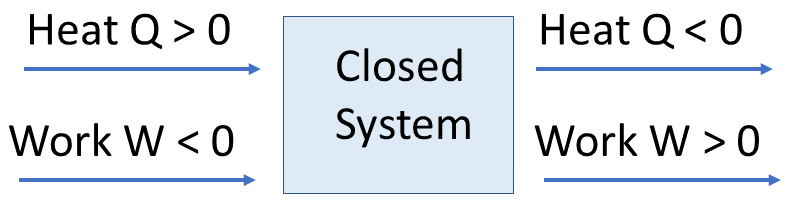
- \( \mathrm{q > 0} \): heat added to the system
- \( \mathrm{q < 0} \): heat lost by the system
- \( \mathrm{W > 0} \): work done on the system (compression)
- \( \mathrm{W < 0} \): work done by the system (expansion)
Physical Meaning:
- Heating increases molecular kinetic and/or potential energy.
- Work done on a gas (compression) increases internal energy.
- Work done by a gas (expansion) decreases internal energy.
Example
A gas absorbs \( \mathrm{500\ J} \) of heat. No work is done on or by the system. Calculate the change in internal energy.
▶️ Answer / Explanation
Use: \( \mathrm{\Delta U = q + W} \)
\( \mathrm{\Delta U = 500 + 0 = 500\ J} \)
The internal energy increases by 500 J.
Example
A gas loses \( \mathrm{300\ J} \) of heat to the surroundings and \( \mathrm{200\ J} \) of work is done on the gas. Calculate the change in internal energy.
▶️ Answer / Explanation
Here, \( \mathrm{q = -300\ J} \) (heat lost) \( \mathrm{W = +200\ J} \) (work done on the system)
\( \mathrm{\Delta U = q + W = -300 + 200 = -100\ J} \)
The internal energy decreases by 100 J.
Example
A gas is compressed at constant pressure. During the process, it is supplied with \( \mathrm{1200\ J} \) of heat and does \( \mathrm{400\ J} \) of work on the surroundings. Calculate the change in internal energy and determine whether the internal energy increases or decreases.
▶️ Answer / Explanation
Identify values:
- \( \mathrm{q = +1200\ J} \) (heat added to the system)
- \( \mathrm{W = -400\ J} \) (work done by the system → negative)
Apply first law:
\( \mathrm{\Delta U = q + W = 1200 – 400 = 800\ J} \)
Internal energy increases by 800 J.
Physically:
The system gains more energy through heating than it loses by doing work, so its internal energy rises.
