CIE AS/A Level Physics 22.1 Energy and momentum of a photon Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 22.1 Energy and momentum of a photon Study Notes – New Syllabus
CIE AS/A Level Physics 22.1 Energy and momentum of a photon Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- understand that electromagnetic radiation has a particulate nature
- understand that a photon is a quantum of electromagnetic energy
- recall and use \( E = hf\)
- use the electronvolt (eV) as a unit of energy
- understand that a photon has momentum and that the momentum is given by \( p = E/c\)
Particulate Nature of Electromagnetic Radiation
Electromagnetic (EM) radiation such as light, X-rays, microwaves, etc.. is commonly described as a wave. However, experiments show that EM radiation also behaves like a stream of particles. These particles are called photons.
Key Idea: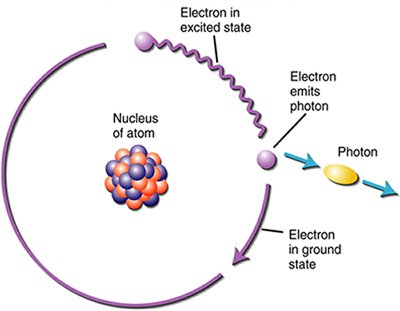
Electromagnetic radiation is made up of discrete packets of energy called photons.
Photon Energy:
\( \mathrm{E = hf} \)
- \( \mathrm{E} \) = energy of one photon
- \( \mathrm{h} \) = Planck’s constant \( (6.63\times10^{-34}\ \mathrm{J\,s}) \)
- \( \mathrm{f} \) = frequency of the radiation
Evidence for the Particulate Nature:
- Photoelectric effect → light ejects electrons one photon at a time.
- X-ray production → high-energy photons emitted during electron deceleration.
- Compton scattering → photons collide with electrons like particles.
Conclusion:
EM radiation exhibits wave–particle duality: it behaves both as a wave and as a particle.
Example
What name is given to the discrete packets of energy that make up electromagnetic radiation?
▶️ Answer / Explanation
Photons. EM radiation behaves like particles called photons, each carrying energy.
Example
Blue light has a higher frequency than red light. Using the particulate model of EM radiation, explain why blue light photons have more energy.
▶️ Answer / Explanation
Photon energy is given by:
\( \mathrm{E = hf} \)
Since blue light has a higher frequency, its photons have larger \( \mathrm{hf} \). Therefore, blue-light photons carry more energy than red-light photons.
Example
A photon of ultraviolet radiation has a frequency of \( \mathrm{9.0\times10^{14}\ Hz} \). Calculate its energy.
▶️ Answer / Explanation
Use:
\( \mathrm{E = hf} \)
\( \mathrm{E = (6.63\times10^{-34})(9.0\times10^{14})} \)
\( \mathrm{E = 5.97\times10^{-19}\ J} \)
Photon energy = \( \mathrm{6.0\times10^{-19}\ J} \)
Photon as a Quantum of Electromagnetic Energy
A photon is the fundamental particle of electromagnetic radiation. It carries energy in a discrete, indivisible packet, known as a quantum.
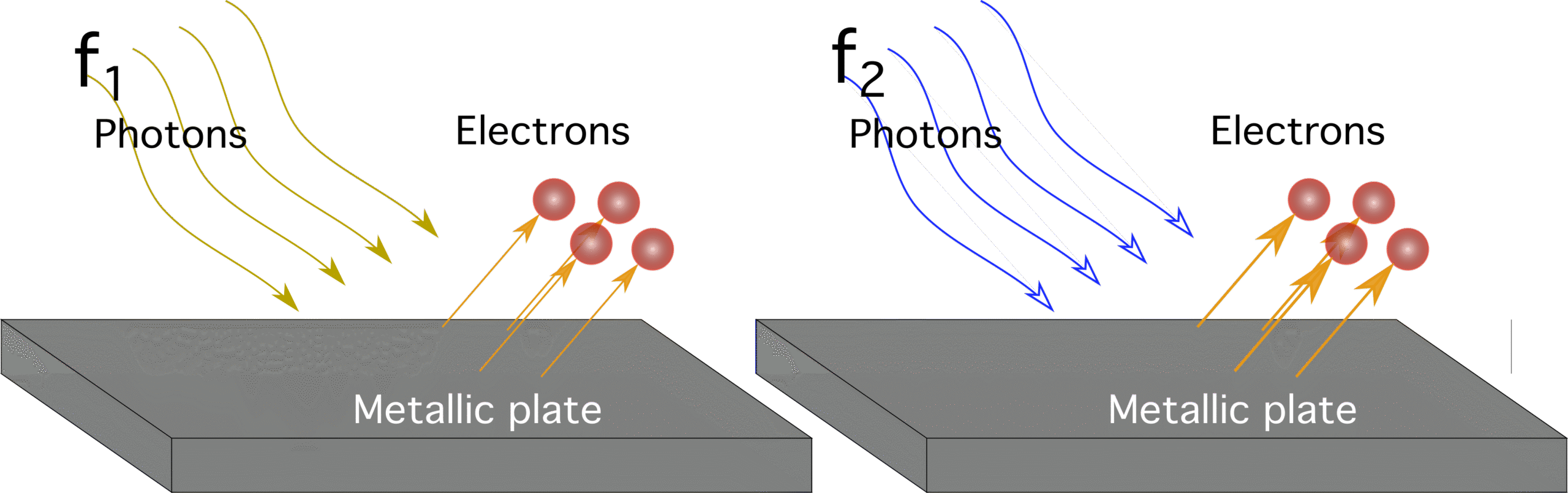
Key Idea:
A photon is a quantum of electromagnetic energy the smallest possible amount of EM energy that can exist or be transferred.
This means:
- EM radiation is not continuous it comes in bundles.
- Each bundle (photon) has a fixed energy that depends on frequency.
Photon Energy:
\( \mathrm{E = hf} \)
- \( \mathrm{E} \) = energy of one photon
- \( \mathrm{h} \) = Planck’s constant
- \( \mathrm{f} \) = frequency of radiation
Implications of Photon Quantisation
- You cannot have “half a photon”.
- Increasing brightness means more photons, not more energetic photons.
- Higher frequency → each photon carries more energy.
Examples of Photon Behaviour:
- Photoelectric effect: electrons are ejected one photon at a time.
- Emission spectra: atoms emit photons of discrete energies.
- Laser light: coherent photons of the same energy.
Example
What is the name given to the smallest packet of electromagnetic energy?
▶️ Answer / Explanation
A photon — it represents one quantum of EM energy.
Example
Explain why increasing the brightness of a light source increases the number of emitted photoelectrons but does not increase their maximum kinetic energy.
▶️ Answer / Explanation
Increasing brightness increases the number of photons arriving per second, not the energy of each photon.
- More photons → more electrons can be ejected.
- Photon energy depends only on frequency: \( \mathrm{E = hf} \).
- Thus brightness does not affect electron kinetic energy.
Only frequency determines the energy of each photon.
Example
A photon of light has a wavelength of \( \mathrm{3.0\times10^{-7}\ m} \). Show that it is a high-energy photon by calculating its energy.
▶️ Answer / Explanation
Photon energy can be written as:
\( \mathrm{E = hf = \dfrac{hc}{\lambda}} \)
Substitute values:
\( \mathrm{E = \dfrac{(6.63\times10^{-34})(3.0\times10^{8})}{3.0\times10^{-7}}} \)
\( \mathrm{E = \dfrac{1.989\times10^{-25}}{3.0\times10^{-7}} = 6.63\times10^{-19}\ J} \)
This is a high-energy photon (UV range).
Using the Photon Energy Equation \( \mathrm{E = hf} \)
The energy of a single photon of electromagnetic radiation is given by:
\( \mathrm{E = hf} \)
- \( \mathrm{E} \) = energy of one photon (J)
- \( \mathrm{h} \) = Planck’s constant \( \mathrm{(6.63\times10^{-34}\ J\,s)} \)
- \( \mathrm{f} \) = frequency of radiation (Hz)
Meaning:
- Photon energy is directly proportional to frequency.
- Higher frequency → higher energy photons (e.g., gamma rays).
- Lower frequency → lower energy photons (e.g., radio waves).
You may also use the alternative form:
\( \mathrm{E = \dfrac{hc}{\lambda}} \)
when wavelength is given.
Example
Calculate the energy of a photon with frequency \( \mathrm{5.0\times10^{14}\ Hz} \).
▶️ Answer / Explanation
Use \( \mathrm{E = hf} \):
\( \mathrm{E = (6.63\times10^{-34})(5.0\times10^{14})} \)
\( \mathrm{E = 3.32\times10^{-19}\ J} \)
Photon energy = \( \mathrm{3.3\times10^{-19}\ J} \)
Example
A photon has energy \( \mathrm{4.0\times10^{-19}\ J} \). Calculate its frequency.
▶️ Answer / Explanation
Rearrange:
\( \mathrm{f = \dfrac{E}{h}} \)
\( \mathrm{f = \dfrac{4.0\times10^{-19}}{6.63\times10^{-34}}} \)
\( \mathrm{f = 6.03\times10^{14}\ Hz} \)
Frequency ≈ \( \mathrm{6.0\times10^{14}\ Hz} \)
Example
Light of wavelength \( \mathrm{250\ nm} \) strikes a surface. Calculate the photon energy using \( \mathrm{E = hf} \).
▶️ Answer / Explanation
Use frequency: \( \mathrm{f = \dfrac{c}{\lambda}} \)
\( \mathrm{f = \dfrac{3.0\times10^{8}}{250\times10^{-9}} = 1.2\times10^{15}\ Hz} \)
Now photon energy:
\( \mathrm{E = hf = (6.63\times10^{-34})(1.2\times10^{15})} \)
\( \mathrm{E = 7.96\times10^{-19}\ J} \)
Photon energy ≈ \( \mathrm{8.0\times10^{-19}\ J} \)
Electronvolt (eV) as a Unit of Energy
The electronvolt (eV) is a small unit of energy commonly used in atomic, nuclear, and particle physics.
Definition: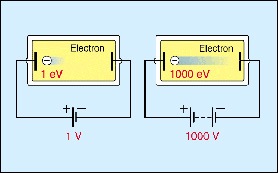
1 electronvolt (1 eV) is the energy gained by an electron when it is accelerated through a potential difference of 1 volt.
In joules:
\( \mathrm{1\ eV = 1.60\times10^{-19}\ J} \)
Why use electronvolts?
- Much more convenient than joules for tiny energies.
- Useful for photon energies, ionisation energies, nuclear transitions.
- Easy to link with potentials in electric fields.
Conversions:
- \( \mathrm{E(J) = E(eV)\times1.60\times10^{-19}} \)
- \( \mathrm{E(eV) = \dfrac{E(J)}{1.60\times10^{-19}}} \)
Example
Convert 5 eV into joules.
▶️ Answer / Explanation
\( \mathrm{E = 5 \times 1.60\times10^{-19} = 8.0\times10^{-19}\ J} \)
Energy = \( \mathrm{8.0\times10^{-19}\ J} \)
Example
A photon has energy \( \mathrm{4.8\times10^{-19}\ J} \). Express this energy in electronvolts.
▶️ Answer / Explanation
Use:
\( \mathrm{E(eV) = \dfrac{E(J)}{1.60\times10^{-19}}} \)
\( \mathrm{E = \dfrac{4.8\times10^{-19}}{1.60\times10^{-19}} = 3.0\ eV} \)
Energy = 3 eV
Example
An electron is accelerated through a potential difference of 2500 V. Calculate its energy gain in:
- (a) electronvolts
- (b) joules
▶️ Answer / Explanation
(a) In eV:
Energy gained = 2500 eV
(b) In joules:
\( \mathrm{E = 2500 \times 1.60\times10^{-19}} \)
\( \mathrm{E = 4.0\times10^{-16}\ J} \)
Energy = \( \mathrm{4.0\times10^{-16}\ J} \)
Photon Momentum and the Relation \( \mathrm{p = \dfrac{E}{c}} \)
Although photons have no rest mass, they still carry momentum. This is a fundamental result of quantum theory and explains effects such as radiation pressure and Compton scattering.
Key Idea:
A photon has momentum even though it has zero rest mass.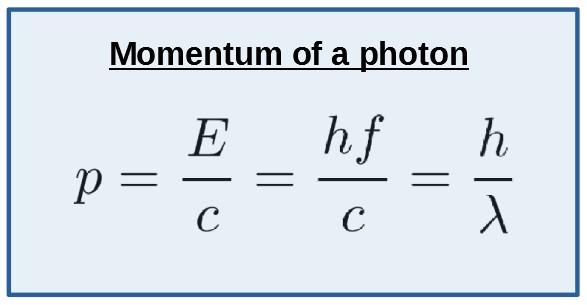
The photon’s momentum is related to its energy by:
\( \mathrm{p = \dfrac{E}{c}} \)
- \( \mathrm{p} \) = photon momentum (kg·m/s)
- \( \mathrm{E} \) = photon energy (J)
- \( \mathrm{c} \) = speed of light \( \mathrm{3.0\times10^8\ m/s} \)
Alternative form using wavelength:
\( \mathrm{p = \dfrac{h}{\lambda}} \)
since \( \mathrm{E = hf = \dfrac{hc}{\lambda}} \).
Why does a photon have momentum?
- Photons carry energy → all energy has momentum associated with it.
- Electromagnetic waves exert pressure (radiation pressure) → requires momentum transfer.
- Explains phenomena such as:
- Compton scattering
- Solar sail propulsion
- Momentum recoil during photon emission
Example
A photon has an energy of \( \mathrm{3.0\times10^{-19}\ J} \). Calculate its momentum.
▶️ Answer / Explanation
Use:
\( \mathrm{p = \dfrac{E}{c}} \)
\( \mathrm{p = \dfrac{3.0\times10^{-19}}{3.0\times10^8} = 1.0\times10^{-27}\ kg\,m/s} \)
Photon momentum = \( \mathrm{1.0\times10^{-27}\ kg\,m/s} \)
Example
What is the momentum of a photon with wavelength \( \mathrm{500\ nm} \)? (Use \( \mathrm{p = \dfrac{h}{\lambda}} \).)
▶️ Answer / Explanation
Convert wavelength:
\( \mathrm{500\ nm = 500\times10^{-9}\ m} \)
Use:
\( \mathrm{p = \dfrac{h}{\lambda} = \dfrac{6.63\times10^{-34}}{500\times10^{-9}}} \)
\( \mathrm{p = 1.33\times10^{-27}\ kg\,m/s} \)
Photon momentum ≈ \( \mathrm{1.3\times10^{-27}\ kg\,m/s} \)
Example
A photon has frequency \( \mathrm{9.0\times10^{14}\ Hz} \). Calculate its momentum using \( \mathrm{p = \dfrac{E}{c}} \).
▶️ Answer / Explanation
Step 1: Find energy using \( \mathrm{E = hf} \).
\( \mathrm{E = (6.63\times10^{-34})(9.0\times10^{14}) = 5.97\times10^{-19}\ J} \)
Step 2: Use momentum formula:
\( \mathrm{p = \dfrac{E}{c} = \dfrac{5.97\times10^{-19}}{3.0\times10^8}} \)
\( \mathrm{p = 1.99\times10^{-27}\ kg\,m/s} \)
Photon momentum ≈ \( \mathrm{2.0\times10^{-27}\ kg\,m/s} \)
