CIE AS/A Level Physics 22.2 Photoelectric effect Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 22.2 Photoelectric effect Study Notes – New Syllabus
CIE AS/A Level Physics 22.2 Photoelectric effect Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- understand that photoelectrons may be emitted from a metal surface when it is illuminated by electromagnetic radiation
- understand and use the terms threshold frequency and threshold wavelength
- explain photoelectric emission in terms of photon energy and work function energy
- recall and use \( hf = \Phi + \tfrac{1}{2} m v_{\text{max}}^{2}\)
- explain why the maximum kinetic energy of photoelectrons is independent of intensity, whereas the photoelectric current is proportional to intensity
Photoelectric Emission
When electromagnetic radiation shines onto the surface of a metal, it can cause electrons to be emitted from that surface. These emitted electrons are called photoelectrons.
Key Idea:
Photoelectrons are emitted from a metal surface when incident electromagnetic radiation transfers enough photon energy to liberate electrons from the metal.
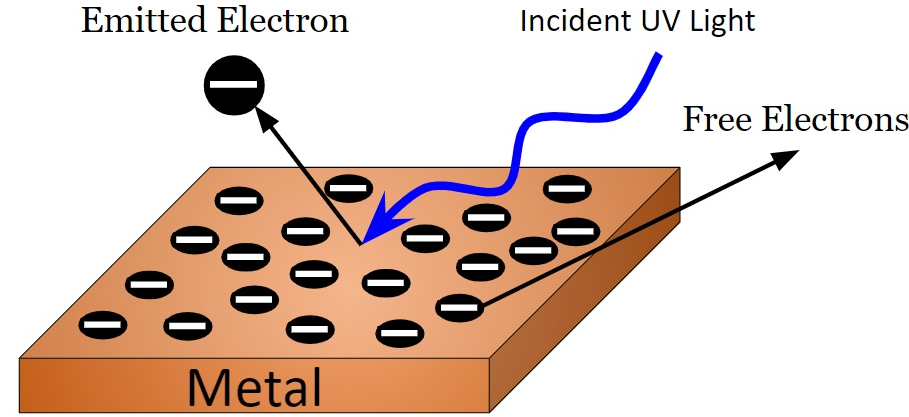
How Photoelectric Emission Occurs
- Each photon carries energy \( \mathrm{E = hf} \).
- If a photon hits a metal electron, it transfers all its energy to that electron.
- If the photon energy is greater than or equal to the metal’s work function, the electron is released.
- The emitted electron is called a photoelectron.
Work Function ( \( \mathrm{\phi} \) ):
The minimum energy needed to free an electron from the metal surface.
Important Notes:
- Low-frequency light (e.g., red light) may not eject electrons at all.
- High-frequency light (e.g., UV) ejects electrons even if intensity is low.
- Increasing intensity increases number of emitted electrons, not the maximum energy.
Summary Statement:
Electromagnetic radiation can eject electrons from metals via the photoelectric effect, provided photon energy exceeds the work function.
Example
Blue light causes electrons to be emitted from a metal surface, but red light does not. What does this indicate?
▶️ Answer / Explanation
Blue light has higher frequency → higher photon energy \( \mathrm{E = hf} \). Red light has lower frequency → insufficient photon energy.
The metal’s work function lies between the energies of red and blue photons.
Example
A metal emits photoelectrons when illuminated by UV light but not when illuminated by visible light of any intensity. Explain why.
▶️ Answer / Explanation
Visible-light photons have insufficient energy to overcome the metal’s work function. Increasing intensity increases the number of photons but not their energy.
UV photons have much higher frequency → higher energy → can eject electrons.
Therefore, only UV radiation exceeds the work function threshold.
Example
A metal has a work function of \( \mathrm{3.2\times10^{-19}\ J} \). Determine whether light of wavelength \( \mathrm{320\ nm} \) can cause photoemission.
▶️ Answer / Explanation
Step 1: Find photon energy:
\( \mathrm{E = \dfrac{hc}{\lambda} = \dfrac{(6.63\times10^{-34})(3.0\times10^8)}{320\times10^{-9}}} \)
\( \mathrm{E = 6.21\times10^{-19}\ J} \)
Step 2: Compare with work function:
- Photon energy = \( \mathrm{6.21\times10^{-19}\ J} \)
- Work function = \( \mathrm{3.2\times10^{-19}\ J} \)
Since \( \mathrm{E > \phi} \), photoelectrons are emitted.
The radiation can eject electrons.
Threshold Frequency and Threshold Wavelength
For photoelectrons to be emitted from a metal surface, the photons must carry enough energy to overcome the metal’s work function \( \mathrm{\phi} \). This leads to two important terms: threshold frequency and threshold wavelength.
Threshold Frequency \( \mathrm{f_0} \)
The threshold frequency is the minimum frequency of electromagnetic radiation required to eject electrons from a metal surface.
It is the frequency at which photon energy equals the work function:
\( \mathrm{hf_0 = \phi} \)
- If \( \mathrm{f < f_0} \): no electrons emitted (no matter how intense the light).
- If \( \mathrm{f \ge f_0} \): photoelectrons are emitted.
Threshold Wavelength \( \mathrm{\lambda_0} \)
The threshold wavelength is the maximum wavelength of radiation that can cause photoemission.
Longer wavelengths have lower frequency and lower photon energy, so they cannot eject electrons.
Using \( \mathrm{c = f\lambda} \), threshold wavelength is:
\( \mathrm{\lambda_0 = \dfrac{c}{f_0}} \)
Or from the work function:
\( \mathrm{\phi = \dfrac{hc}{\lambda_0}} \)
- If \( \mathrm{\lambda > \lambda_0} \): photon energy too small → no emission.
- If \( \mathrm{\lambda \le \lambda_0} \): electrons are emitted.
Example
What happens if light of frequency lower than the threshold frequency strikes a metal surface?
▶️ Answer / Explanation
The photon energy is less than the work function. No electrons are emitted, regardless of intensity.
Example
A metal has a threshold frequency of \( \mathrm{7.0\times10^{14}\ Hz} \). Calculate its threshold wavelength.
▶️ Answer / Explanation
Use:
\( \mathrm{\lambda_0 = \dfrac{c}{f_0}} \)
\( \mathrm{\lambda_0 = \dfrac{3.0\times10^{8}}{7.0\times10^{14}} = 4.29\times10^{-7}\ m} \)
Threshold wavelength = \( \mathrm{429\ nm} \)
Example
A metal has a work function of \( \mathrm{4.5\times10^{-19}\ J} \). Calculate:
- (a) the threshold frequency
- (b) the threshold wavelength
▶️ Answer / Explanation
(a) Threshold frequency:
\( \mathrm{hf_0 = \phi} \Rightarrow \mathrm{f_0 = \dfrac{\phi}{h}} \)
\( \mathrm{f_0 = \dfrac{4.5\times10^{-19}}{6.63\times10^{-34}} = 6.79\times10^{14}\ Hz} \)
(b) Threshold wavelength:
\( \mathrm{\lambda_0 = \dfrac{c}{f_0}} \)
\( \mathrm{\lambda_0 = \dfrac{3.0\times10^{8}}{6.79\times10^{14}} = 4.42\times10^{-7}\ m} \)
Threshold wavelength = \( \mathrm{442\ nm} \)
Photoelectric Emission in Terms of Photon Energy and Work Function
The photoelectric effect occurs when electromagnetic radiation shines on a metal surface and causes electrons to be emitted. This can be fully explained using the concepts of photon energy and work function energy.
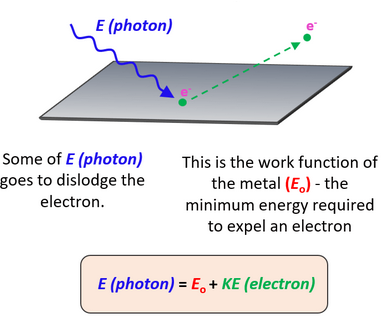
Photon Energy
\( \mathrm{E = hf} \)
- Each photon carries a discrete amount of energy \( \mathrm{E} \).
- Energy depends only on frequency \( \mathrm{f} \), not on brightness.
Work Function Energy \( \mathrm{\phi} \)
The work function is the minimum energy required to remove an electron from the surface of a metal.
- If photon energy is too small → electron cannot escape.
- \( \mathrm{\phi} \) depends on the metal.
Condition for Photoelectric Emission
Photoelectrons are emitted only if: \( \mathrm{hf \geq \phi} \)
- If \( \mathrm{hf < \phi} \): no electrons emitted (even at high intensity).
- If \( \mathrm{hf = \phi} \): electrons just escape with zero kinetic energy.
- If \( \mathrm{hf > \phi} \): electrons are emitted with kinetic energy.
Maximum Kinetic Energy of Emitted Electrons
\( \mathrm{K_{max} = hf – \phi} \)
Interpretation:
- Photon energy (input) = work function + kinetic energy.
- Any excess energy becomes electron kinetic energy.
Example
Light of frequency \( \mathrm{f} \) shines on a metal but does not eject electrons. What does this say about the photon energy?
▶️ Answer / Explanation
The photons have insufficient energy to overcome the work function.
\( \mathrm{hf < \phi} \)
No photoelectrons are emitted.
Example
A photon has energy \( \mathrm{5.0\times10^{-19}\ J} \) and the metal’s work function is \( \mathrm{3.0\times10^{-19}\ J} \). Explain why electrons are emitted and calculate their maximum kinetic energy.
▶️ Answer / Explanation
Since:
\( \mathrm{hf = 5.0\times10^{-19} > \phi = 3.0\times10^{-19}} \)
Photoemission occurs.
Maximum kinetic energy:
\( \mathrm{K_{max} = hf – \phi = 5.0\times10^{-19} – 3.0\times10^{-19}} \)
\( \mathrm{K_{max} = 2.0\times10^{-19}\ J} \)
Example
Light of wavelength \( \mathrm{280\ nm} \) strikes a metal with work function \( \mathrm{4.0\times10^{-19}\ J} \). Determine whether electrons are emitted, and if so, find their maximum kinetic energy.
▶️ Answer / Explanation
Step 1: Calculate photon energy:
\( \mathrm{E = \dfrac{hc}{\lambda}} \)
\( \mathrm{E = \dfrac{(6.63\times10^{-34})(3.0\times10^8)}{280\times10^{-9}}} \)
\( \mathrm{E = 7.10\times10^{-19}\ J} \)
Step 2: Compare with work function:
- Photon energy = \( \mathrm{7.10\times10^{-19}} \)
- Work function = \( \mathrm{4.0\times10^{-19}} \)
Since \( \mathrm{E > \phi} \), electrons are emitted.
Maximum kinetic energy:
\( \mathrm{K_{max} = 7.10\times10^{-19} – 4.0\times10^{-19}} \)
\( \mathrm{K_{max} = 3.10\times10^{-19}\ J} \)
Electrons are emitted with maximum kinetic energy \( \mathrm{3.1\times10^{-19}\ J} \).
Photoelectric Equation: \( \mathrm{hf = \Phi + \tfrac{1}{2}mv_{\text{max}}^{2}} \)
This is the fundamental energy equation for the photoelectric effect. It relates the energy of an incoming photon to the work needed to remove an electron from the metal and the maximum kinetic energy of the emitted electron.
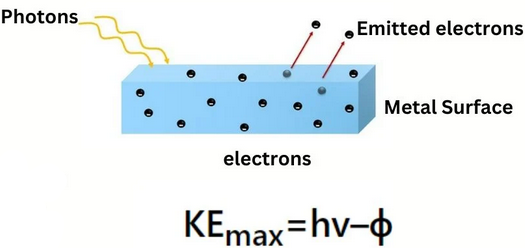
Meaning of Each Term
- \( \mathrm{hf} \) → energy of one photon
- \( \mathrm{\Phi} \) → work function (minimum energy to release an electron)
- \( \mathrm{\tfrac{1}{2}mv_{\text{max}}^{2}} \) → maximum kinetic energy of emitted electrons
The equation:
\( \mathrm{hf = \Phi + \tfrac{1}{2}mv_{\text{max}}^{2}} \)
Interpretation:
- Photon energy (input) is used to:
- overcome the binding energy of the metal (work function)
- give kinetic energy to the emitted electron
- If \( \mathrm{hf = \Phi} \): electrons just escape → zero kinetic energy.
- If \( \mathrm{hf > \Phi} \): electrons escape with kinetic energy.
- If \( \mathrm{hf < \Phi} \): no emission, regardless of intensity.
Example
A photon has energy \( \mathrm{6.0\times10^{-19}\ J} \). The metal’s work function is \( \mathrm{4.0\times10^{-19}\ J} \). Calculate the maximum kinetic energy of the emitted electron.
▶️ Answer / Explanation
Use:
\( \mathrm{K_{max} = hf – \Phi} \)
\( \mathrm{K_{max} = 6.0\times10^{-19} – 4.0\times10^{-19}} \)
\( \mathrm{K_{max} = 2.0\times10^{-19}\ J} \)
Maximum kinetic energy = \( \mathrm{2.0\times10^{-19}\ J} \)
Example
Light of frequency \( \mathrm{7.5\times10^{14}\ Hz} \) shines on a metal with work function \( \mathrm{3.6\times10^{-19}\ J} \). Calculate the maximum speed of the emitted electrons.
▶️ Answer / Explanation
Step 1: Photon energy
\( \mathrm{hf = (6.63\times10^{-34})(7.5\times10^{14}) = 4.97\times10^{-19}\ J} \)
Step 2: Kinetic energy
\( \mathrm{K_{max} = hf – \Phi = 4.97\times10^{-19} – 3.6\times10^{-19}} \)
\( \mathrm{K_{max} = 1.37\times10^{-19}\ J} \)
Step 3: Use \( \mathrm{K = \tfrac{1}{2}mv^2} \)
\( \mathrm{v = \sqrt{\dfrac{2K}{m}} = \sqrt{\dfrac{2(1.37\times10^{-19})}{9.11\times10^{-31}}}} \)
\( \mathrm{v = 5.48\times10^{5}\ m/s} \)
Maximum electron speed ≈ \( \mathrm{5.5\times10^{5}\ m/s} \)
Example
Ultraviolet light of wavelength \( \mathrm{260\ nm} \) strikes a metal. The work function of the metal is \( \mathrm{3.9\times10^{-19}\ J} \). Calculate the maximum kinetic energy and maximum speed of the photoelectrons.
▶️ Answer / Explanation
Step 1: Photon energy
\( \mathrm{E = \dfrac{hc}{\lambda}} \)
\( \mathrm{E = \dfrac{(6.63\times10^{-34})(3.0\times10^8)}{260\times10^{-9}}} \)
\( \mathrm{E = 7.65\times10^{-19}\ J} \)
Step 2: Maximum kinetic energy
\( \mathrm{K_{max} = E – \Phi = 7.65\times10^{-19} – 3.9\times10^{-19}} \)
\( \mathrm{K_{max} = 3.75\times10^{-19}\ J} \)
Step 3: Maximum speed
\( \mathrm{v = \sqrt{\dfrac{2K}{m}} = \sqrt{\dfrac{2(3.75\ttimes10^{-19})}{9.11\times10^{-31}}}} \)
\( \mathrm{v = 9.08\times10^{5}\ m/s} \)
Maximum KE = \( \mathrm{3.75\times10^{-19}\ J} \)
Maximum speed ≈ \( \mathrm{9.1\times10^{5}\ m/s} \)
Why Maximum Kinetic Energy Is Independent of Intensity, but Photoelectric Current Is Proportional to Intensity
The behaviour of photoelectrons in the photoelectric effect can be fully explained using the photon model of light.
1. Maximum Kinetic Energy Depends on Photon Energy
Each emitted electron absorbs energy from one photon only. Photon energy is: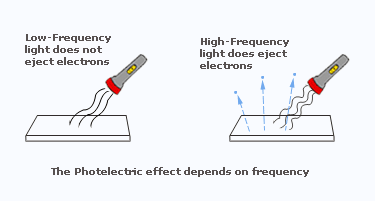
\( \mathrm{E = hf} \)
So:
- Higher frequency → higher photon energy → higher kinetic energy of electrons.
- Intensity does not change photon energy.
Therefore:
The maximum kinetic energy of photoelectrons depends only on frequency, not intensity.
This is because intensity changes the number of photons, not the energy per photon.
2. Photoelectric Current Depends on Number of Photoelectrons
Intensity of light means: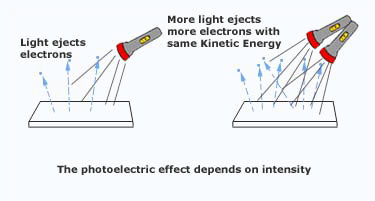
More intensity → more photons per second.
Since each photon can release one electron:
- More photons → more emitted electrons per second.
- More electrons per second → larger photoelectric current.
Therefore:
Photoelectric current is directly proportional to intensity.
3. Summary of the Reasoning
| Quantity | Depends on | Reason |
|---|---|---|
| Maximum kinetic energy | Frequency only | One electron absorbs energy from one photon → energy = hf |
| Photoelectric current | Intensity | Intensity controls number of photons → number of electrons emitted |
Example
Shining brighter (higher intensity) blue light on a metal increases the current but not the maximum electron speed. Explain why.
▶️ Answer / Explanation
Brighter light → more photons → more electrons emitted → higher current.
The energy of each photon is unchanged because frequency is unchanged, so:
\( \mathrm{K_{max}} \) stays the same.
Example
Two beams of ultraviolet light have the same frequency. Beam A has twice the intensity of Beam B. Compare the maximum kinetic energy of the emitted electrons from each beam.
▶️ Answer / Explanation
- Both beams have the same frequency → same photon energy.
- Therefore, maximum kinetic energy \( \mathrm{K_{max}} \) is identical.
- Beam A will produce twice the current because it has twice the number of photons.
Example
A light source of frequency \( \mathrm{8.0\times10^{14}\ Hz} \) produces photoelectrons with maximum kinetic energy \( \mathrm{1.2\times10^{-19}\ J} \). If the intensity is doubled at the same frequency, predict the new maximum kinetic energy and the change in current.
▶️ Answer / Explanation
Maximum kinetic energy:
Depends only on frequency → unchanged.
New \( \mathrm{K_{max}} = 1.2\times10^{-19}\ J}
Photoelectric current:
Doubling intensity → doubles number of photons → doubles number of emitted electrons.
New current = double the original current.
