CIE AS/A Level Physics 22.3 Wave-particle duality Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 22.3 Wave-particle duality Study Notes – New Syllabus
CIE AS/A Level Physics 22.3 Wave-particle duality Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- understand that the photoelectric effect provides evidence for a particulate nature of electromagnetic radiation while phenomena such as interference and diffraction provide evidence for a wave nature
- describe and interpret qualitatively the evidence provided by electron diffraction for the wave nature of particles
- understand the de Broglie wavelength as the wavelength associated with a moving particle
- recall and use \( \lambda = h/p \)
Wave–Particle Duality: Evidence from Photoelectric Effect and Interference/Diffraction
Electromagnetic radiation (light, X-rays, microwaves, etc.) shows behaviour of both particles and waves. This dual nature is demonstrated by two key sets of experiments:
- Photoelectric effect → particle behaviour (photons)
- Interference & diffraction → wave behaviour
1. Evidence for Particulate Nature: The Photoelectric Effect
In the photoelectric effect, electrons are emitted from a metal surface when electromagnetic radiation shines on it.
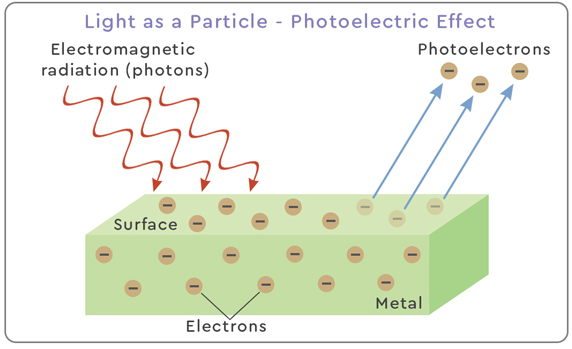
Key observations that prove light behaves like particles:
- Electrons are emitted only if light frequency is above a threshold — regardless of intensity.
- Emission is instantaneous even at low intensities → energy must come in discrete packets.
- Maximum kinetic energy of electrons depends on frequency, not intensity.
Explanation:
Light delivers energy in discrete packets called photons.
Each photon has energy \( \mathrm{E = hf} \). If this energy exceeds the work function, an electron is emitted.
This behaviour cannot be explained by a classical wave model.
2. Evidence for Wave Nature: Interference and Diffraction
Light passing through slits or edges spreads out and overlaps, forming characteristic patterns.
- Interference: bright and dark fringes from superposition of waves.
- Diffraction: spreading of waves when passing through a narrow opening.
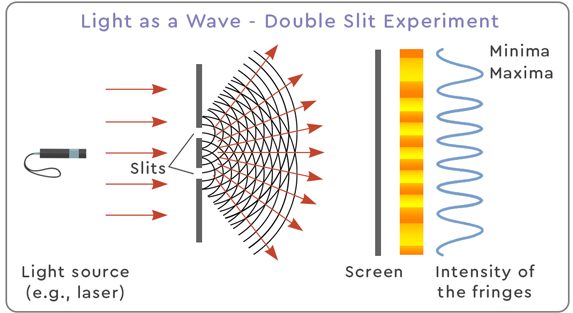
These effects require a wave model:
- Constructive interference → waves in phase.
- Destructive interference → waves out of phase.
- Diffraction increases when slit width ≈ wavelength.
Particles cannot produce these interference/diffraction patterns.
Conclusion: Wave–Particle Duality
Light behaves as a particle (photon) in photoelectric emission and as a wave in diffraction and interference.
This dual behaviour demonstrates the fundamental quantum nature of electromagnetic radiation.
Example
Why does the photoelectric effect support a particle theory rather than a wave theory of light?
▶️ Answer / Explanation
A wave would deliver energy gradually, but electrons are emitted instantaneously only when photon energy exceeds the work function.
This shows energy comes in discrete packets → particles (photons).
Example
Explain why interference fringes are considered evidence for the wave nature of light.
▶️ Answer / Explanation
Interference occurs when waves superpose, producing alternating bright and dark fringes.
Only waves can interfere in this way → therefore interference proves light behaves as a wave.
Example
Describe an experiment showing both particle and wave behaviour and explain how it supports wave–particle duality.
▶️ Answer / Explanation
Experiment: Low-intensity double-slit with single photons
- Photons are sent one at a time → detected individually → particle behaviour.
- Over time, an interference pattern builds up → wave behaviour.
Interpretation:
- Each photon behaves like a particle when detected.
- But its probability distribution behaves like a wave.
This demonstrates that electromagnetic radiation has both wave and particle properties.
Electron Diffraction as Evidence for the Wave Nature of Particles
Electron diffraction experiments provide direct and powerful evidence that particles can behave like waves. This supports the concept of wave–particle duality introduced by de Broglie.
Key Idea:
When a beam of electrons passes through a thin crystal or through narrow gaps, it produces a diffraction pattern something only waves can do.
This shows that electrons (normally thought of as particles) also have a wavelength and can exhibit wave behaviour.
Setup of the Electron Diffraction Experiment
- A beam of high-speed electrons is directed at a thin graphite crystal or nickel crystal.
- The atoms in the crystal form regularly spaced layers that act like a diffraction grating.
- On a screen, a pattern of bright and dark rings or spots is observed.
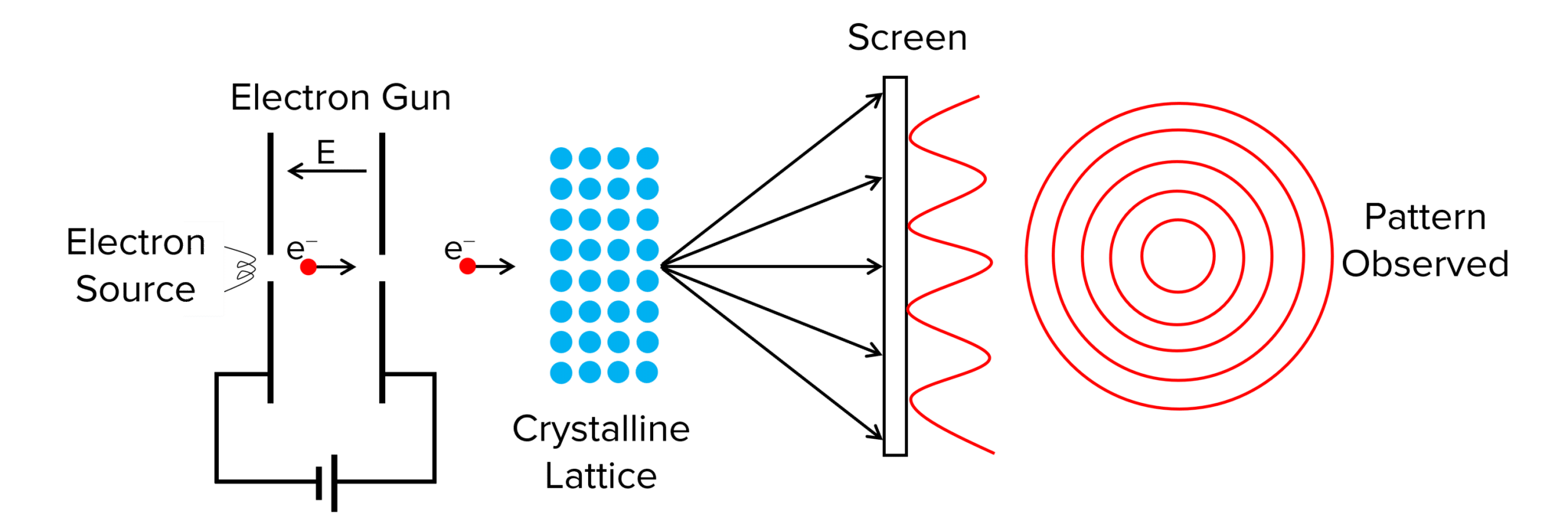
This ring pattern is identical to diffraction patterns produced by x-rays (waves).
Why This Proves Electrons Behave as Waves
Only waves can:
- interfere constructively (bright rings/spots)
- interfere destructively (dark rings/spots)
- diffract when passing through small structures
Therefore, electrons must have a wavelength given by the de Broglie relation:
\( \mathrm{\lambda = \dfrac{h}{p}} \)
When electron momentum decreases (lower speed), the wavelength increases → diffraction becomes more pronounced.
This behaviour is purely wave-like.
Qualitative Interpretation of the Evidence
- Electrons form diffraction rings → electrons behave like waves.
- Changing the electron speed changes the diameter of the diffraction rings → wavelength depends on momentum.
- The phenomenon cannot be explained using classical particle theory.
Conclusion:
Electron diffraction confirms that particles such as electrons have wave properties.
Example
Why does observing diffraction of electrons provide evidence that electrons behave as waves?
▶️ Answer / Explanation
Diffraction is a property of waves, not classical particles. When electrons produce a diffraction pattern, this shows they must have a wavelength and behave like waves.
Example
In an electron diffraction experiment, the diffraction rings become wider when the accelerating voltage is decreased. Explain this observation qualitatively.
▶️ Answer / Explanation
Lower accelerating voltage → electrons move slower → smaller momentum.
Since \( \mathrm{\lambda = h/p} \), wavelength increases.
Larger wavelength causes greater diffraction → wider rings.
This shows electrons behave like waves with variable wavelength.
Example
Explain how the similarity between x-ray diffraction patterns and electron diffraction patterns supports de Broglie’s hypothesis.
▶️ Answer / Explanation
x-rays are waves and produce diffraction patterns when passing through a crystal lattice.
Electrons produce the same type of ring pattern when accelerated to high speeds and directed at a crystal.
Therefore, electrons must also behave like waves with a wavelength comparable to atomic spacing.
This directly supports de Broglie’s hypothesis that particles have wave-like properties with wavelength:
\( \mathrm{\lambda = \dfrac{h}{p}} \)
The de Broglie Wavelength of a Moving Particle
The de Broglie hypothesis states that all moving particles electrons, protons, atoms, even large objects have an associated wavelength. This idea unifies wave and particle behaviour and is central to quantum mechanics.
Definition: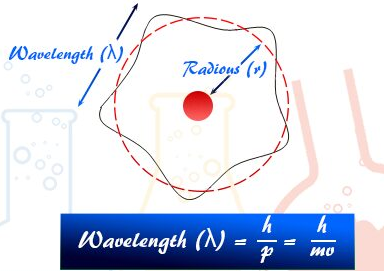
The de Broglie wavelength is the wavelength associated with a particle due to its motion.
It is given by the formula:
\( \mathrm{\lambda = \dfrac{h}{p}} \)
- \( \mathrm{\lambda} \) = de Broglie wavelength (m)
- \( \mathrm{h} \) = Planck’s constant \( \mathrm{6.63\times10^{-34}\ J\,s} \)
- \( \mathrm{p} \) = momentum of the particle \( \mathrm{(mv)} \)
Key Idea:
Faster particles have more momentum → shorter wavelength. Slower particles have less momentum → longer wavelength.
This explains why low-energy electrons produce stronger diffraction patterns than high-energy electrons.
Wave–Particle Duality Interpretation
- Particles can behave like waves if their wavelength is similar to the size of the structure they interact with.
- Electrons have measurable de Broglie wavelengths because their mass is small → momentum is small.
- Larger objects (e.g., tennis balls) have extremely tiny de Broglie wavelengths → no observable wave effects.
Example
What happens to the de Broglie wavelength of a particle if its speed increases?
▶️ Answer / Explanation
Momentum increases → wavelength decreases.
So the de Broglie wavelength becomes shorter.
Example
An electron has momentum \( \mathrm{3.0\times10^{-24}\ kg\,m/s} \). Calculate its de Broglie wavelength.
▶️ Answer / Explanation
Use:
\( \mathrm{\lambda = \dfrac{h}{p}} \)
\( \mathrm{\lambda = \dfrac{6.63\times10^{-34}}{3.0\times10^{-24}}} \)
\( \mathrm{\lambda = 2.21\times10^{-10}\ m} \)
De Broglie wavelength ≈ \( \mathrm{0.22\ nm} \)
Example
A neutron of mass \( \mathrm{1.67\times10^{-27}\ kg} \) moves at \( \mathrm{2.5\times10^{3}\ m/s} \). Calculate its de Broglie wavelength.
▶️ Answer / Explanation
Step 1: Calculate momentum:
\( \mathrm{p = mv = (1.67\times10^{-27})(2.5\times10^{3}) = 4.18\times10^{-24}\ kg\,m/s} \)
Step 2: Use the de Broglie formula:
\( \mathrm{\lambda = \dfrac{6.63\times10^{-34}}{4.18\times10^{-24}}} \)
\( \mathrm{\lambda = 1.59\times10^{-10}\ m} \)
De Broglie wavelength ≈ \( \mathrm{0.16\ nm} \)
Using the de Broglie Relation \( \mathrm{\lambda = \dfrac{h}{p}} \)
The de Broglie equation gives the wavelength associated with any moving particle. It shows that matter has wave-like properties, with wavelength depending on momentum.
de Broglie Equation: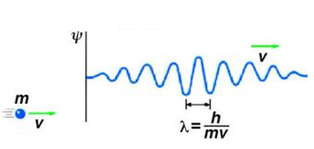
\( \mathrm{\lambda = \dfrac{h}{p}} \)
- \( \mathrm{\lambda} \) = de Broglie wavelength (m)
- \( \mathrm{h} \) = Planck’s constant = \( \mathrm{6.63\times10^{-34}\ J\,s} \)
- \( \mathrm{p} \) = momentum of particle = \( \mathrm{mv} \)
Key understanding:
- Higher momentum → shorter wavelength.
- Lower momentum → longer wavelength.
- Wave properties become detectable only if the wavelength is comparable to atomic spacing (~\( \mathrm{10^{-10}\ m} \)).
Example
A particle’s momentum doubles. What happens to its de Broglie wavelength?
▶️ Answer / Explanation
Since \( \mathrm{\lambda = \dfrac{h}{p}} \), wavelength is inversely proportional to momentum.
Momentum doubles → wavelength halves.
Example
An electron has momentum \( \mathrm{2.0\times10^{-24}\ kg\,m/s} \). Calculate its de Broglie wavelength.
▶️ Answer / Explanation
Use:
\( \mathrm{\lambda = \dfrac{h}{p} = \dfrac{6.63\times10^{-34}}{2.0\times10^{-24}}} \)
\( \mathrm{\lambda = 3.32\times10^{-10}\ m} \)
de Broglie wavelength = \( \mathrm{0.332\ nm} \)
Example
A proton of mass \( \mathrm{1.67\times10^{-27}\ kg} \) is moving at \( \mathrm{1.0\times10^{5}\ m/s} \). Calculate its de Broglie wavelength.
▶️ Answer / Explanation
Step 1: Momentum
\( \mathrm{p = mv = (1.67\times10^{-27})(1.0\times10^{5}) = 1.67\times10^{-22}\ kg\,m/s} \)
Step 2: de Broglie wavelength
\( \mathrm{\lambda = \dfrac{6.63\times10^{-34}}{1.67\times10^{-22}}} \)
\( \mathrm{\lambda = 3.97\times10^{-12}\ m} \)
de Broglie wavelength ≈ \( \mathrm{4.0\times10^{-12}\ m} \)
