CIE AS/A Level Physics 22.4 Energy levels in atoms and line spectra Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 22.4 Energy levels in atoms and line spectra Study Notes – New Syllabus
CIE AS/A Level Physics 22.4 Energy levels in atoms and line spectra Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- understand that there are discrete electron energy levels in isolated atoms (e.g. atomic hydrogen)
- understand the appearance and formation of emission and absorption line spectra
- recall and use \( hf = E_1 – E_2 \)
Discrete Electron Energy Levels in Isolated Atoms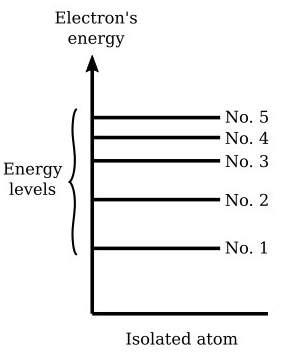
Electrons in atoms cannot possess any arbitrary amount of energy. Instead, they are restricted to discrete (quantised) energy levels, especially in simple atoms like hydrogen.
Key Idea:
Electrons in an isolated atom can only occupy certain fixed energy levels they cannot exist between these levels.
These levels are usually labelled:
- \( \mathrm{n = 1} \): ground state (lowest energy)
- \( \mathrm{n = 2, 3, 4, \dots} \): excited states (higher energy)
The energy of each level is negative (bound state), and becomes less negative as \( \mathrm{n} \) increases.
For the hydrogen atom, the energy levels are given by:
\( \mathrm{E_n = -\dfrac{13.6\ eV}{n^2}} \)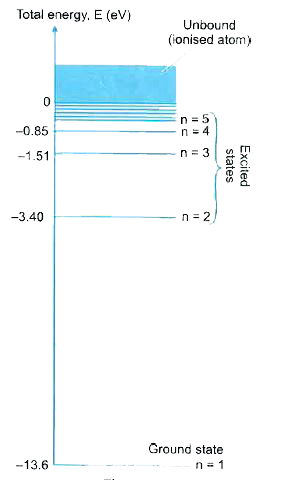
This shows that only specific energy values are allowed.
Important Consequences of Discrete Energy Levels
- An electron can move between levels only by absorbing or emitting a photon.
- The photon energy must match exactly the energy difference between levels.
- This leads to the formation of discrete spectral lines in emission or absorption spectra.
- No continuous energy values are allowed → evidence of quantisation.
Why are energy levels discrete?
- The electron behaves like a standing wave around the nucleus.
- Only certain wavelengths (and therefore energies) form stable standing wave states.
- Therefore, each permitted orbit/state corresponds to a specific allowed energy.
Example
What does it mean that the energy levels in hydrogen are quantised?
▶️ Answer / Explanation
The electron can only exist in specific allowed energy states and cannot have energy values between these states.
Transitions between levels occur only by absorbing or emitting fixed photon energies.
Example
An electron in hydrogen moves from \( \mathrm{n = 3} \) to \( \mathrm{n = 2} \). Explain why a specific wavelength of light is emitted.
▶️ Answer / Explanation
Only fixed energy levels are allowed. The energy difference:
\( \mathrm{\Delta E = E_2 – E_3} \)
is a single unique value.
Thus the emitted photon has energy:
\( \mathrm{E = h f} \)
so only one specific frequency (and wavelength) is produced. That is why hydrogen’s emission spectrum has discrete lines.
Example
Hydrogen energy levels are given by \( \mathrm{E_n = -\dfrac{13.6}{n^2} \ eV} \). Calculate the energy required to excite an electron from \( \mathrm{n = 1} \) to \( \mathrm{n = 3} \), and explain why only that exact energy works.
▶️ Answer / Explanation
Step 1: Find energies
\( \mathrm{E_1 = -13.6\ eV} \)
\( \mathrm{E_3 = -\dfrac{13.6}{9} = -1.51\ eV} \)
Step 2: Energy needed for excitation
\( \mathrm{\Delta E = E_3 – E_1 = (-1.51) – (-13.6)} \)
\( \mathrm{\Delta E = 12.09\ eV} \)
Interpretation
- The photon must supply exactly \( \mathrm{12.09\ eV} \).
- If the photon has less energy → electron cannot reach level 3.
- If more → energy cannot be partially absorbed → no transition occurs.
This demonstrates that energy levels are discrete.
Emission and Absorption Line Spectra
Atoms have discrete electron energy levels. When electrons move between these levels, they absorb or emit photons of specific energies. This leads to the formation of line spectra, which are powerful evidence of quantised atomic energy levels.
Emission Line Spectra
An emission line spectrum consists of bright, coloured lines on a dark background.
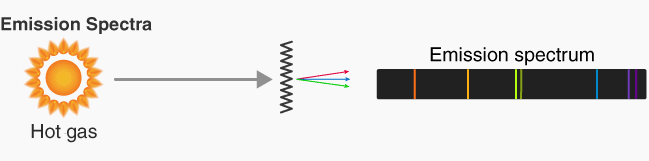
How it is formed:
- Electrons in excited states fall to lower energy levels.
- They release energy as photons.
- The photon energy equals the difference between two energy levels:
\( \mathrm{E = hf = E_{high} – E_{low}} \)
- Each transition produces a photon of a specific wavelength.
- Only certain wavelengths appear → bright lines.
Appearance:
- Bright coloured lines at specific wavelengths.
- Each element produces a unique pattern → “atomic fingerprint”.
Absorption Line Spectra
An absorption line spectrum consists of dark lines on an otherwise continuous spectrum.
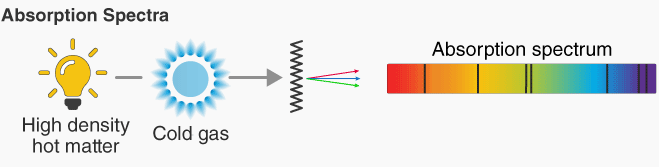
How it is formed:
- White light passes through a cool gas of atoms.
- Electrons absorb photons of specific energies and move to higher levels.
- Only photons with energies matching energy level gaps are absorbed.
- These wavelengths are removed from the spectrum.
Appearance:
- A continuous spectrum with dark lines at specific wavelengths.
- Dark lines correspond to the missing absorbed wavelengths.
Key Relationship Between the Two Spectra
- Emission lines occur at the same wavelengths as absorption lines.
- Both correspond to energy differences between atomic levels.
Same transitions → same photon energies → same wavelengths.
Example
Why does a hydrogen discharge tube produce an emission line spectrum instead of a continuous spectrum?
▶️ Answer / Explanation
Hydrogen atoms have discrete energy levels. Electrons falling from higher to lower levels emit photons of only certain energies → only specific wavelengths appear → bright lines.
Example
White light passes through a cold hydrogen gas. Why are dark lines observed at very specific wavelengths in the output spectrum?
▶️ Answer / Explanation
Electrons in hydrogen atoms absorb only photons whose energies match energy level gaps. These wavelengths are removed from the continuous light, producing dark absorption lines.
Example
Explain why the wavelengths of absorption lines for hydrogen in starlight match exactly the wavelengths of the emission lines produced by a hydrogen lamp in a laboratory.
▶️ Answer / Explanation
Both absorption and emission involve transitions between the same quantised energy levels:
\( \mathrm{\Delta E = E_{high} – E_{low}} \)
In emission, this energy is released as a photon. In absorption, the same amount of energy is taken in to excite an electron.
Therefore, the wavelengths must be identical because they correspond to the same energy differences.
Using the Relation \( \mathrm{hf = E_1 – E_2} \)
This equation links the energy of a photon to the difference between two electron energy levels in an atom. It is the basis of understanding atomic spectra.
Meaning of the Equation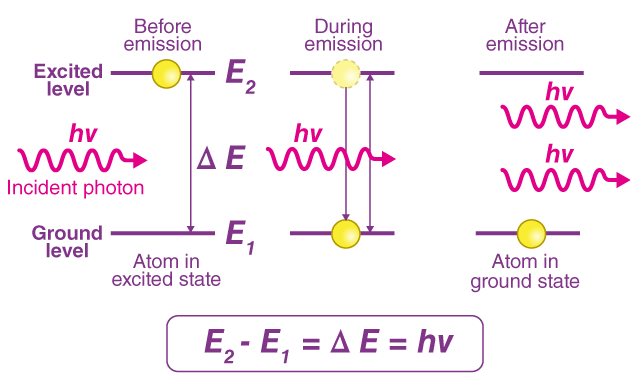
\( \mathrm{hf = E_1 – E_2} \)
- \( \mathrm{h} \) = Planck’s constant
- \( \mathrm{f} \) = frequency of emitted or absorbed photon
- \( \mathrm{E_1} \) = higher energy level
- \( \mathrm{E_2} \) = lower energy level
Key idea:
- If an electron falls from a higher level to a lower level → a photon is emitted.
- If an electron absorbs a photon and jumps to a higher level → a photon is absorbed.
The energy of that photon is exactly equal to the energy difference between the two levels.
Photon Wavelength Form
Using \( \mathrm{c = f\lambda} \):
\( \mathrm{\lambda = \dfrac{hc}{E_1 – E_2}} \)
Example
An electron drops from energy level \( \mathrm{E_1 = -3.4\ eV} \) to \( \mathrm{E_2 = -13.6\ eV} \). Calculate the photon energy emitted in eV.
▶️ Answer / Explanation
\( \mathrm{\Delta E = E_1 – E_2 = (-3.4) – (-13.6) = 10.2\ eV} \)
Photon energy = 10.2 eV
Example
A photon of frequency \( \mathrm{5.0\times10^{14}\ Hz} \) is emitted when an electron falls between two levels. Calculate the energy difference \( \mathrm{E_1 – E_2} \).
▶️ Answer / Explanation
Use:
\( \mathrm{hf = E_1 – E_2} \)
\( \mathrm{E = (6.63\times10^{-34})(5.0\times10^{14})} \)
\( \mathrm{E = 3.32\times10^{-19}\ J} \)
Energy difference = \( \mathrm{3.32\times10^{-19}\ J} \)
Example
Hydrogen has energy levels \( \mathrm{E_2 = -3.4\ eV} \), \( \mathrm{E_5 = -0.544\ eV} \). Calculate the wavelength of the photon emitted when an electron falls from \( \mathrm{n=5} \) to \( \mathrm{n=2} \).
▶️ Answer / Explanation
Step 1: Energy difference
\( \mathrm{\Delta E = E_5 – E_2 = (-0.544) – (-3.4) = 2.856\ eV} \)
Convert to joules:
\( \mathrm{E = 2.856\times1.60\times10^{-19} = 4.57\times10^{-19}\ J} \)
Step 2: Use wavelength formula
\( \mathrm{\lambda = \dfrac{hc}{E}} \)
\( \mathrm{\lambda = \dfrac{(6.63\times10^{-34})(3.0\times10^8)}{4.57\times10^{-19}}} \)
\( \mathrm{\lambda = 4.36\times10^{-7}\ m} \)
Wavelength = \( \mathrm{436\ nm} \)
