CIE AS/A Level Physics 23.1 Mass defect and nuclear binding energy Study Notes- 2025-2027 Syllabus
CIE AS/A Level Physics 23.1 Mass defect and nuclear binding energy Study Notes – New Syllabus
CIE AS/A Level Physics 23.1 Mass defect and nuclear binding energy Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Physics latest syllabus with Candidates should be able to:
- understand the equivalence between energy and mass as represented by \( E = mc^2\) and recall and use this equation
- represent simple nuclear reactions by nuclear equations of the form \( \mathrm{{}^{14}_{7}N + {}^{4}_{2}He \rightarrow {}^{17}_{8}O + {}^{1}_{1}H} \)
- define and use the terms mass defect and binding energy
- sketch the variation of binding energy per nucleon with nucleon number
- explain what is meant by nuclear fusion and nuclear fission
- explain the relevance of binding energy per nucleon to nuclear reactions, including nuclear fusion and nuclear fission
- calculate the energy released in nuclear reactions using \( E = c^2 \Delta m\)
Mass–Energy Equivalence and the Equation \( \mathrm{E = mc^2} \)
The famous equation proposed by Einstein states that mass and energy are equivalent and can be converted into one another. This relationship is fundamental in nuclear physics and particle physics.
Meaning of the Equation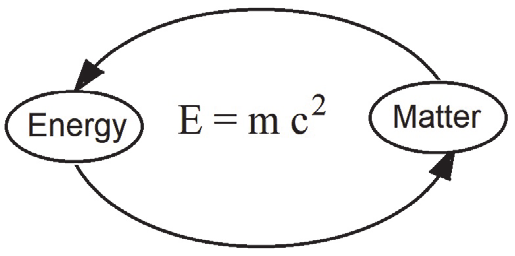
\( \mathrm{E = mc^2} \)
- \( \mathrm{E} \) = energy (J)
- \( \mathrm{m} \) = mass (kg)
- \( \mathrm{c} \) = speed of light in vacuum \( \mathrm{3.0\times10^8\ m/s} \)
Key idea:
A small amount of mass corresponds to a huge amount of energy because \( \mathrm{c^2} \) is extremely large.
Physical Interpretation
- Mass can be “converted” into energy (e.g., nuclear fusion, fission).
- Energy can appear as additional mass (e.g., binding energy of nuclei).
- Mass is not conserved alone → mass–energy together is conserved.
- Even an object at rest has rest energy \( \mathrm{E_0 = mc^2} \).
Using the Equation
To find the energy equivalent of a mass:
\( \mathrm{E = mc^2} \)
To find the mass equivalent of energy:
\( \mathrm{m = \dfrac{E}{c^2}} \)
Example
Find the energy equivalent of a mass of \( \mathrm{2.0\times10^{-5}\ kg} \).
▶️ Answer / Explanation
\( \mathrm{E = mc^2 = (2.0\times10^{-5})(3.0\times10^8)^2} \)
\( \mathrm{E = 2.0\times10^{-5}\times9.0\times10^{16}} \)
\( \mathrm{E = 1.8\times10^{12}\ J} \)
Energy = \( \mathrm{1.8\times10^{12}\ J} \)
Example
If a process releases \( \mathrm{3.0\times10^{13}\ J} \) of energy, calculate the mass lost.
▶️ Answer / Explanation
Use:
\( \mathrm{m = \dfrac{E}{c^2}} \)
\( \mathrm{m = \dfrac{3.0\times10^{13}}{(3.0\times10^8)^2}} \)
\( \mathrm{m = \dfrac{3.0\times10^{13}}{9.0\times10^{16}} = 3.33\times10^{-4}\ kg} \)
Mass lost = \( \mathrm{3.3\times10^{-4}\ kg} \)
Example
A nuclear reaction reduces the mass of a nucleus by \( \mathrm{5.0\times10^{-30}\ kg} \). Calculate the energy released, and state whether this is likely to be large or small.
▶️ Answer / Explanation
Energy released:
\( \mathrm{E = mc^2 = (5.0\times10^{-30})(3.0\times10^8)^2} \)
\( \mathrm{E = 5.0\times10^{-30}\times9.0\times10^{16}} \)
\( \mathrm{E = 4.5\times10^{-13}\ J} \)
Interpretation:
- This is a tiny amount of mass.
- But the energy is large for such a small mass → due to \( \mathrm{c^2} \) being huge.
- This explains why nuclear reactions release enormous energy.
Representing Simple Nuclear Reactions Using Nuclear Equations
Nuclear reactions involve changes in the nucleus of an atom. These reactions must obey two conservation laws:
- Conservation of nucleon number (mass number A)
- Conservation of proton number (atomic number Z)
These rules allow us to write correct nuclear equations of the form:
\( \mathrm{{}^{14}_{7}N + {}^{4}_{2}He \rightarrow {}^{17}_{8}O + {}^{1}_{1}H} \)
Each term is written as:
\( \mathrm{{}^{A}_{Z}X} \quad \) where A = nucleon number (protons + neutrons) Z = proton (atomic) number X = chemical symbol
How to Construct a Nuclear Equation
- Add the mass numbers (A) on the left and ensure the same total appears on the right.
- Add the atomic numbers (Z) on the left and ensure the same total appears on the right.
- Identify any unknown particle using its A and Z values.
Example
Complete the nuclear equation:
\( \mathrm{{}^{14}_{6}C \rightarrow {}^{14}_{7}N + \ ? } \)
▶️ Answer / Explanation
Step 1: Mass number
Left: 14 → Right: 14 + A ⇒ A = 0
Step 2: Atomic number
Left: 6 → Right: 7 + Z ⇒ Z = –1
The particle with \( \mathrm{A = 0, Z = -1} \) is a beta particle (\( \mathrm{\beta^-} \)).
Final equation:
\( \mathrm{{}^{14}_{6}C \rightarrow {}^{14}_{7}N + {}^{0}_{-1}\beta} \)
Example
Write the nuclear equation for the alpha decay of polonium-210.
Alpha particle = \( \mathrm{{}^{4}_{2}He} \)
▶️ Answer / Explanation
Start with:
\( \mathrm{{}^{210}_{84}Po \rightarrow {}^{4}_{2}He + X} \)
Conserve mass number:
210 = 4 + A ⇒ A = 206
Conserve atomic number:
84 = 2 + Z ⇒ Z = 82
Element with Z = 82 is lead (Pb).
Final equation:
\( \mathrm{{}^{210}_{84}Po \rightarrow {}^{206}_{82}Pb + {}^{4}_{2}He} \)
Example
Complete the following nuclear reaction equation:
\( \mathrm{{}^{27}_{13}Al + {}^{4}_{2}He \rightarrow X + {}^{1}_{0}n } \)
▶️ Answer / Explanation
Step 1: Mass number balance
Left: \( \mathrm{27 + 4 = 31} \) Right: \( \mathrm{A + 1 = 31} ⇒ A = 30\)
Step 2: Atomic number balance
Left: \( \mathrm{13 + 2 = 15} \) Right: \( \mathrm{Z + 0 = 15} ⇒ Z = 15\)
Element with Z = 15 is phosphorus (P).
Final equation:
\( \mathrm{{}^{27}_{13}Al + {}^{4}_{2}He \rightarrow {}^{30}_{15}P + {}^{1}_{0}n } \)
Mass Defect and Binding Energy
Atomic nuclei are made of protons and neutrons, yet the mass of a nucleus is always less than the sum of the masses of its separate nucleons. This missing mass is called the mass defect and corresponds to the binding energy that holds the nucleus together.
Mass Defect (Δm)
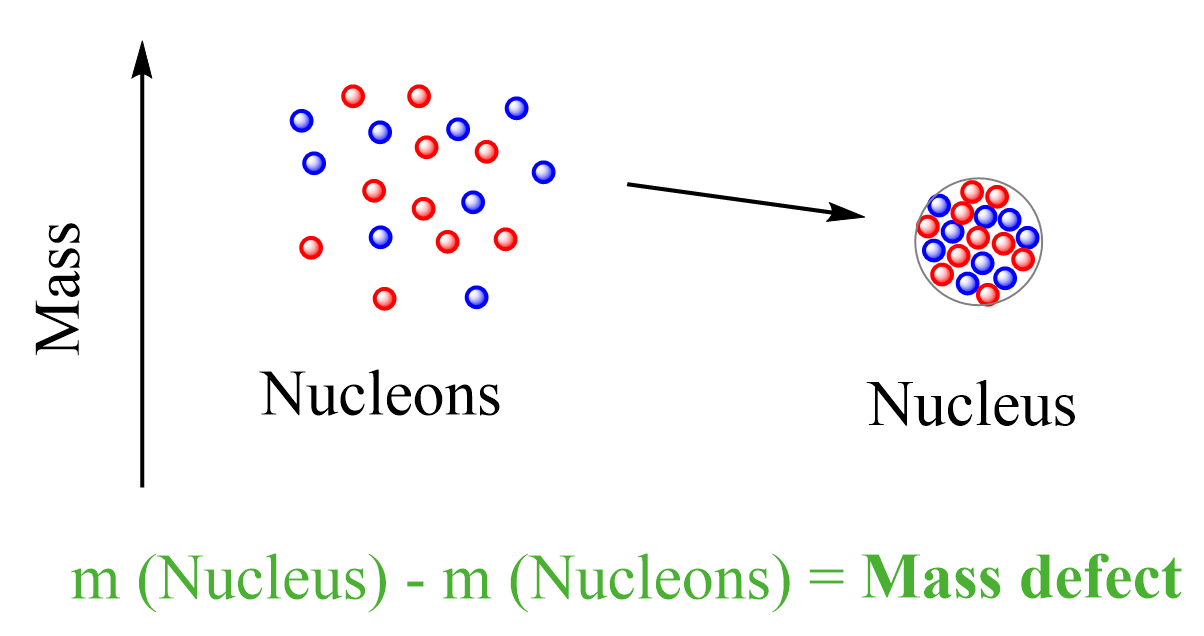
The mass defect is the difference between the total mass of the individual nucleons and the actual mass of the nucleus.
\( \mathrm{\Delta m = (Z m_p + N m_n) – m_{nucleus}} \)
- \( \mathrm{Z} \): number of protons
- \( \mathrm{N} \): number of neutrons
- \( \mathrm{m_p} \): mass of one proton
- \( \mathrm{m_n} \): mass of one neutron
- \( \mathrm{m_{nucleus}} \): measured mass of the nucleus
This “missing mass” has been converted into binding energy.
Binding Energy (Eb)
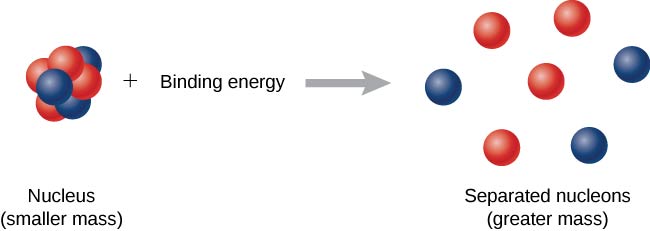
The binding energy is the energy required to separate a nucleus into its individual protons and neutrons.
Relation between mass defect and binding energy:
\( \mathrm{E_b = \Delta m \, c^2} \)
This comes directly from mass–energy equivalence.
Binding Energy per Nucleon:
A measure of nuclear stability — higher value means a more stable nucleus.
Example
In a nucleus, the sum of the masses of individual nucleons is greater than the measured mass of the nucleus. What is this difference called?
▶️ Answer / Explanation
This difference is the mass defect, which corresponds to the binding energy that holds the nucleus together.
Example
For a nucleus, the total mass of separate nucleons is \( \mathrm{3.00\times10^{-26}\ kg} \), and the actual mass of the nucleus is \( \mathrm{2.99\times10^{-26}\ kg} \). Calculate the mass defect.
▶️ Answer / Explanation
\( \mathrm{\Delta m = 3.00\times10^{-26} – 2.99\times10^{-26}} \)
\( \mathrm{\Delta m = 1.0\times10^{-28}\ kg} \)
Mass defect = \( \mathrm{1.0\times10^{-28}\ kg} \)
Example
A nucleus has a mass defect of \( \mathrm{4.0\times10^{-29}\ kg} \). Calculate the binding energy and express it in joules.
▶️ Answer / Explanation
Use:
\( \mathrm{E_b = \Delta m \, c^2} \)
\( \mathrm{E_b = (4.0\times10^{-29})(3.0\times10^{8})^2} \)
\( \mathrm{E_b = 4.0\times10^{-29}\times9.0\times10^{16}} \)
\( \mathrm{E_b = 3.6\times10^{-12}\ J} \)
Binding energy = \( \mathrm{3.6\times10^{-12}\ J} \)
Variation of Binding Energy per Nucleon with Nucleon Number
The binding energy per nucleon measures how tightly the nucleus is held together. When plotted against nucleon number \( \mathrm{A} \), it produces one of the most important graphs in nuclear physics.
Qualitative Shape of the Graph
You should be able to sketch and describe the curve. It has the following features:
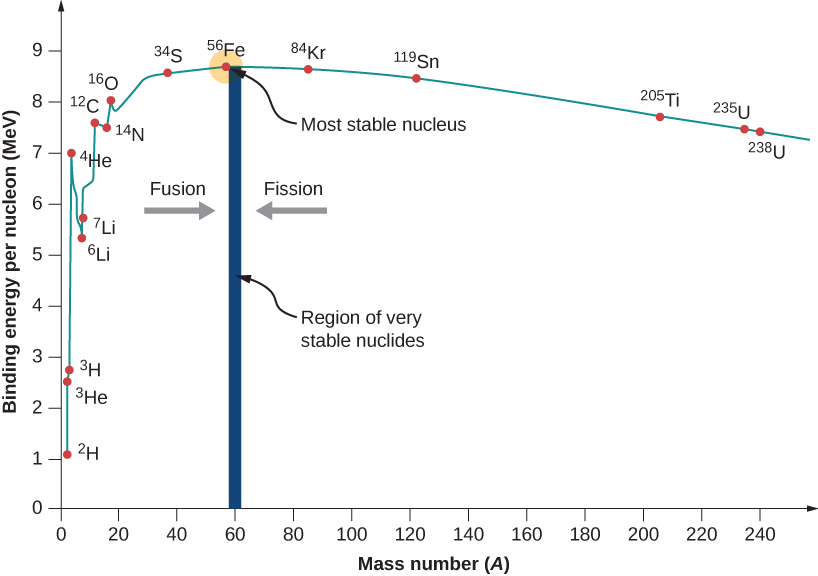
- Very low for the smallest nuclei (e.g., hydrogen, helium).
- Rapid increase up to a peak around \( \mathrm{A \approx 56} \) (iron–56).
- Iron-56 has the highest binding energy per nucleon, meaning it is the most stable nucleus.
- For heavier nuclei (A > 60), the value gradually decreases.
- Heavy nuclei like uranium have much lower binding energy per nucleon → less stable.
Key Regions to Sketch
- Light nuclei (A ≈ 1–20): steep rise.
- Mid-mass nuclei (A ≈ 20–60): curve flattens and peaks at iron.
- Heavy nuclei (A ≥ 60): slow decrease.
Interpretation of the Shape
- Fusion of light nuclei → increases binding energy per nucleon → energy released.
- Fission of heavy nuclei → also increases binding energy per nucleon → energy released.
- Most stable nuclei lie near iron.
How to Sketch It in an Exam
When asked to draw the curve, a good sketch includes:
- x-axis: nucleon number \( \mathrm{A} \)
- y-axis: binding energy per nucleon (MeV)
- Steep rise for small A
- Peak at iron (A ≈ 56)
- Gentle decline for heavy nuclei
Example
Where on the graph does the most stable nucleus appear?
▶️ Answer / Explanation
The most stable nucleus is iron-56, which appears at the peak of the binding energy per nucleon graph.
It has the highest binding energy per nucleon (~8.8 MeV).
Example
Explain why fusion of light nuclei releases energy using the binding energy graph.
▶️ Answer / Explanation
Light nuclei lie on the left-hand rising side of the graph. When two light nuclei fuse, the product nucleus has a higher binding energy per nucleon.
The increase in binding energy means energy is released.
Example
Why does fission of uranium-235 release energy according to the shape of the curve?
▶️ Answer / Explanation
Uranium-235 lies on the right-hand declining part of the curve (lower binding energy per nucleon).
When it splits into medium-sized nuclei (A ≈ 100), the products lie closer to the peak of the curve where binding energy per nucleon is higher.
The increase in binding energy per nucleon results in the release of large amounts of energy.
Nuclear Fusion and Nuclear Fission
Nuclear fusion and nuclear fission are two different nuclear processes that release energy due to changes in binding energy per nucleon. They are explained using the binding energy curve and the idea that nuclei tend to move toward higher stability.
Nuclear Fusion 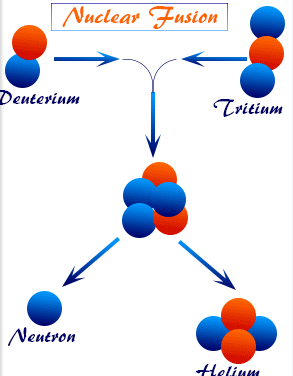
Nuclear fusion is the process in which two light nuclei combine to form a heavier nucleus, releasing energy.
Key points:
- Occurs with light nuclei (e.g., hydrogen isotopes).
- The product nucleus has a higher binding energy per nucleon.
- The increase in binding energy is released as energy.
- Responsible for energy production in stars (e.g., proton–proton cycle).
- Requires extremely high temperatures and pressures to overcome electrostatic repulsion.
General form:
\( \mathrm{{}^{2}_{1}H + {}^{3}_{1}H \rightarrow {}^{4}_{2}He + {}^{1}_{0}n} \)
Nuclear Fission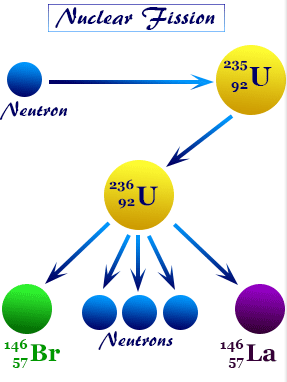
Nuclear fission is the process in which a heavy nucleus splits into two smaller nuclei, releasing energy.
Key points:
- Occurs with very heavy nuclei (e.g., uranium-235, plutonium-239).
- The fission products have higher binding energy per nucleon.
- The increase in binding energy leads to energy release.
- Often triggered by neutron absorption.
- Can produce chain reactions (basis of nuclear reactors and atomic bombs).
General form:
\( \mathrm{{}^{235}_{92}U + {}^{1}_{0}n \rightarrow {}^{141}_{56}Ba + {}^{92}_{36}Kr + 3\,{}^{1}_{0}n} \)
Example
Which process involves the joining of light nuclei: fusion or fission?
▶️ Answer / Explanation
Fusion involves the joining of light nuclei. Fission is the splitting of heavy nuclei.
Example
Explain why nuclear fusion releases energy using the binding energy curve.
▶️ Answer / Explanation
Light nuclei lie on the left rising part of the binding energy per nucleon graph. When they fuse, the product nucleus has a higher binding energy per nucleon. The increase in binding energy is released as energy.
Example
A uranium-235 nucleus undergoes fission and splits into medium-sized nuclei. Explain why this releases energy.
▶️ Answer / Explanation
Uranium-235 lies on the right descending part of the binding energy curve, where binding energy per nucleon is relatively low.
Its fission products (A ≈ 90–140) lie closer to iron-56, at higher binding energy per nucleon. The increase in binding energy per nucleon corresponds to a release of energy.
Relevance of Binding Energy per Nucleon to Nuclear Reactions (Fusion and Fission)
The binding energy per nucleon is a measure of how tightly the nucleons (protons and neutrons) are held together in a nucleus. It determines whether a nuclear reaction will release energy or require energy.
Key Principle
A nuclear reaction releases energy if the total binding energy per nucleon of the products is greater than that of the reactants.
Because a higher binding energy per nucleon means a more stable nucleus, nuclei tend to move toward the region of higher stability on the binding energy curve.
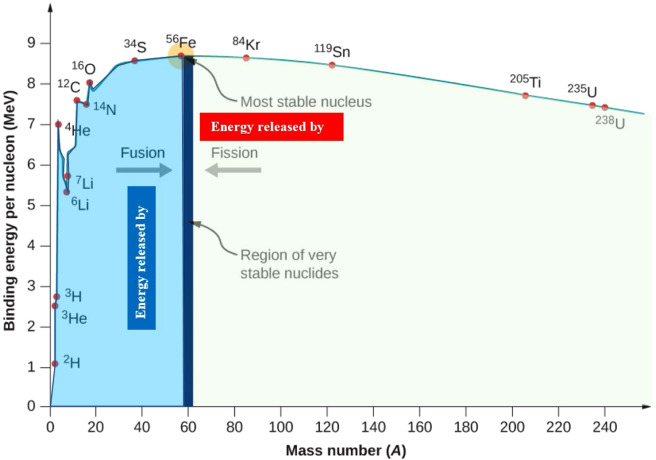
Binding Energy Curve Summary
- Light nuclei (A ≈ 1–20): low binding energy per nucleon.
- Medium nuclei (A ≈ 20–60): highest stability, peak at iron-56.
- Heavy nuclei (A > 60): decreasing binding energy per nucleon.
Relevance to Nuclear Fusion
Fusion is the joining of two light nuclei to form a heavier nucleus.
- Light nuclei lie on the left side of the binding energy curve where binding energy per nucleon is low.
- Fusion produces a nucleus with higher binding energy per nucleon.
- The increase in binding energy becomes released energy.
- This is why fusion powers the Sun and stars.
Essential idea: Fusion is energetically favourable for A < 56.
Relevance to Nuclear Fission
Fission is the splitting of a heavy nucleus into two medium-sized nuclei.
- Heavy nuclei lie on the right side of the binding energy curve where binding energy per nucleon is low.
- Fission produces nuclei closer to the peak of the curve (A ≈ 90–140).
- The products have greater binding energy per nucleon.
- The increase in binding energy is released as .
Essential idea: Fission is energetically favourable for A > 56.
Importance in Nuclear Energy
- Fusion and fission both release energy because they move nuclei toward the highest binding energy region.
- The amount of energy released depends on how much the binding energy per nucleon increases.
- This explains why nuclear processes release far more energy than chemical ones.
Example
Which has higher binding energy per nucleon: a light nucleus such as helium-4 or a medium nucleus such as iron-56?
▶️ Answer / Explanation
Iron-56 has a much higher binding energy per nucleon than helium-4. This makes iron-56 one of the most stable nuclei.
Example
Explain why fusion of hydrogen nuclei releases energy using the binding energy curve.
▶️ Answer / Explanation
Hydrogen nuclei lie on the far left of the curve with low binding energy per nucleon. When they fuse into helium, the binding energy per nucleon increases. This increase in binding energy is released as energy.
Example
Using the binding energy curve, explain why uranium-235 undergoes fission and why this process releases large amounts of energy.
▶️ Answer / Explanation
Uranium-235 lies on the right-hand declining side of the curve, where binding energy per nucleon is relatively low. When it splits into mid-mass nuclei (A ≈ 90–140), the products lie closer to the peak at iron-56, where binding energy per nucleon is higher.
The increase in binding energy per nucleon corresponds to energy released. Because the change is large, fission produces enormous amounts of energy.
Calculating the Energy Released in Nuclear Reactions Using \( \mathrm{E = c^2 \Delta m} \)
Nuclear reactions such as fusion and fission involve very small changes in mass. This “missing mass” (mass defect) is converted into energy according to the mass–energy equivalence equation:
\( \mathrm{E = \Delta m \, c^2} \)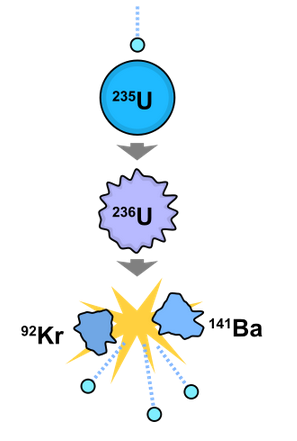
- \( \mathrm{E} \) = energy released (J)
- \( \mathrm{\Delta m} \) = mass defect (kg)
- \( \mathrm{c} \) = speed of light \( \mathrm{3.0\times10^8\ m/s} \)
Key idea:
Even a tiny mass defect produces enormous energy because \( \mathrm{c^2} \) is extremely large.
Steps for Solving Problems
- Find mass of reactants.
- Find mass of products.
- Calculate mass defect: \( \mathrm{\Delta m = m_{reactants} – m_{products}} \)
- Convert to kilograms if needed.
- Use \( \mathrm{E = \Delta m \, c^2} \).
Example
A nuclear reaction has a mass defect of \( \mathrm{1.0\times10^{-30}\ kg} \). Calculate the energy released.
▶️ Answer / Explanation
\( \mathrm{E = \Delta m\,c^2 = (1.0\times10^{-30})(3.0\times10^{8})^2} \)
\( \mathrm{E = 1.0\times10^{-30}\times9.0\times10^{16}} \)
\( \mathrm{E = 9.0\times10^{-14}\ J} \)
Energy released = \( \mathrm{9.0\times10^{-14}\ J} \)
Example
The total mass of reactants in a fusion reaction is \( \mathrm{5.008\times10^{-27}\ kg} \). The mass of the products is \( \mathrm{5.004\times10^{-27}\ kg} \). Calculate the energy released.
▶️ Answer / Explanation
Step 1: Mass defect
\( \mathrm{\Delta m = 5.008\times10^{-27} – 5.004\times10^{-27}} \)
\( \mathrm{\Delta m = 4.0\times10^{-30}\ kg} \)
Step 2: Use \( \mathrm{E = \Delta m \, c^2} \)
\( \mathrm{E = 4.0\times10^{-30}\times9.0\times10^{16}} \)
\( \mathrm{E = 3.6\times10^{-13}\ J} \)
Energy released = \( \mathrm{3.6\times10^{-13}\ J} \)
Example
A fission reaction of uranium-235 produces a mass defect of \( \mathrm{0.200\ u} \). Calculate the energy released. (Use \( \mathrm{1\ u = 1.66\times10^{-27}\ kg} \))
▶️ Answer / Explanation
Step 1: Convert mass defect to kg
\( \mathrm{\Delta m = 0.200\times1.66\times10^{-27}} \)
\( \mathrm{\Delta m = 3.32\times10^{-28}\ kg} \)
Step 2: Use \( \mathrm{E = \Delta m \, c^2} \)
\( \mathrm{E = (3.32\times10^{-28})(9.0\times10^{16})} \)
\( \mathrm{E = 2.99\times10^{-11}\ J} \)
Energy released ≈ \( \mathrm{3.0\times10^{-11}\ J} \)
This is a very large amount of energy from a tiny amount of mass → basis of nuclear power.
