CIE IGCSE Physics (0625) Density Study Notes - New Syllabus
CIE IGCSE Physics (0625) Density Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of Density
Key Concepts:
- Determining Density of Liquids and Solids
Definition of Density
Definition of Density
Density is defined as the mass per unit volume of a substance or object.
- It tells us how tightly packed the matter is in a given volume.
- It is a measure of how heavy a material is for its size.

Formula for Density:
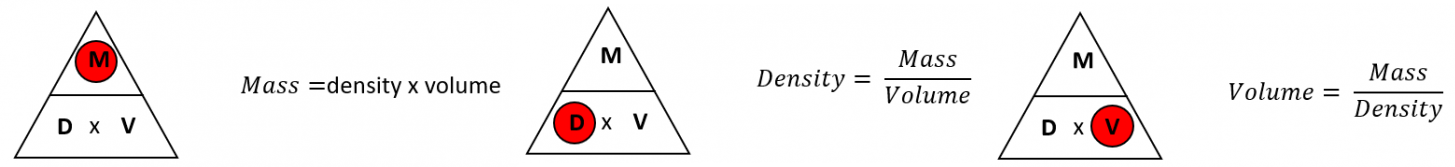
\( \rho = \dfrac{m}{V} \)
- \( \rho \) = density (in kg/m³ or g/cm³)
- \( m \) = mass (in kg or g)
- \( V \) = volume (in m³ or cm³)
SI unit of density: kilogram per cubic metre (kg/m³)
Other common unit: gram per cubic centimetre (g/cm³)
Conversion: 1 g/cm³ = 1000 kg/m³
Applications of density:
- Used to identify substances (e.g. gold vs brass)
- Used in floating/sinking behavior in fluids
- Used in materials science and construction
Example:
A metal block has a mass of 400 g and a volume of 100 cm³. Find its density in g/cm³ and kg/m³.
▶️ Answer/Explanation
Step 1: Use \( \rho = \dfrac{m}{V} \)
\( \rho = \dfrac{400}{100} = 4~\text{g/cm}^3 \)
Step 2: Convert to kg/m³
\( 4~\text{g/cm}^3 = 4 \times 1000 = \boxed{4000~\text{kg/m}^3} \)
Example:
The density of a liquid is 1.2 g/cm³. A sample has a volume of 250 cm³. What is its mass?
▶️ Answer/Explanation
Rearrange: \( m = \rho \cdot V \)
\( m = 1.2 \times 250 = \boxed{300~\text{g}} \)
Determining Density of Liquids and Solids
Determining Density of Liquids and Solids
To calculate density:
Use the formula: \( \rho = \dfrac{m}{V} \)
You must measure:
- Mass — using a digital balance (in g or kg)
- Volume — using appropriate methods for different materials
A. Density of a Liquid
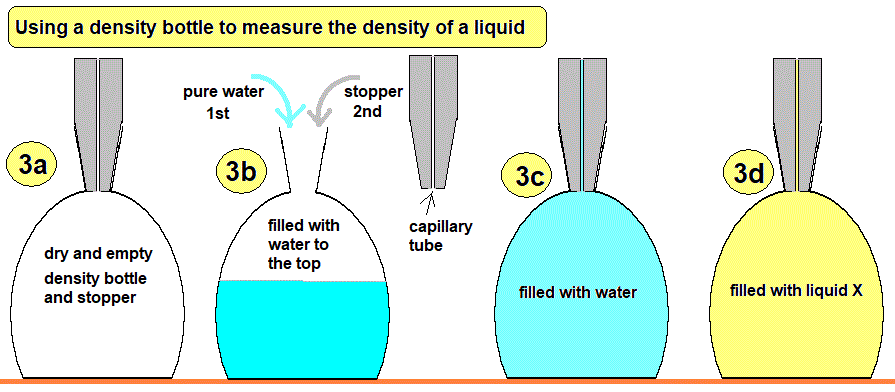
- Use a measuring cylinder to measure a known volume of the liquid (in cm³ or mL).
- Measure the mass of an empty beaker using a digital balance.
- Pour the liquid into the beaker and measure the total mass.
- Subtract to find the mass of the liquid:
\( m_{\text{liquid}} = m_{\text{beaker + liquid}} – m_{\text{beaker}} \)
Then use: \( \rho = \dfrac{m}{V} \)
B. Density of a Regularly Shaped Solid
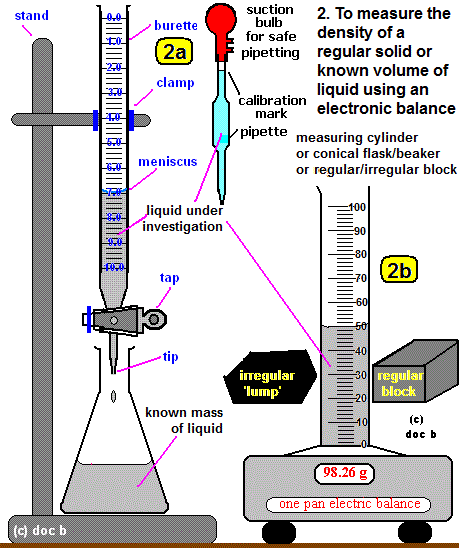
- Measure the mass using a digital balance.
- Measure the dimensions using a ruler or caliper:
- For a cuboid: \( V = \text{length} \times \text{width} \times \text{height} \)
- For a cylinder: \( V = \pi r^2 h \)
- Use the volume in the density formula: \( \rho = \dfrac{m}{V} \)
C. Density of an Irregularly Shaped Solid (via Displacement Method)

- Measure the mass of the solid using a balance.
- Fill a measuring cylinder or overflow can with water to a known level.
- Gently lower the object into the water and note the volume of water displaced.
- This displaced volume = volume of the object (in cm³).
- Use: \( \rho = \dfrac{m}{V} \)
Example:
A student pours 100 cm³ of oil into a beaker. The mass of the beaker is 250 g. The mass of the beaker with oil is 340 g. Find the density of the oil.
▶️ Answer/Explanation
Mass of oil: \( 340 – 250 = 90~\text{g} \)
Volume: \( 100~\text{cm}^3 \)
\( \rho = \dfrac{90}{100} = \boxed{0.9~\text{g/cm}^3} \)
Example:
A wooden block has dimensions 10 cm × 5 cm × 2 cm and a mass of 200 g. Find its density.
▶️ Answer/Explanation
Volume: \( V = 10 \times 5 \times 2 = 100~\text{cm}^3 \)
\( \rho = \dfrac{200}{100} = \boxed{2.0~\text{g/cm}^3} \)
Example:
A wooden block has dimensions 10 cm × 5 cm × 2 cm and a mass of 200 g. Find its density.
▶️ Answer/Explanation
Volume: \( V = 10 \times 5 \times 2 = 100~\text{cm}^3 \)
\( \rho = \dfrac{200}{100} = \boxed{2.0~\text{g/cm}^3} \)
Floating and Sinking Based on Density
Floating and Sinking Based on Density:
- Whether an object floats or sinks in a fluid (liquid or gas) depends on its density compared to the fluid’s density.
Floating Rule:
- If an object’s density is less than the density of the fluid, it will float.
- If an object’s density is greater than the density of the fluid, it will sink.

Explanation:
- Fluids exert an upward force called upthrust or buoyant force.
- If the upthrust equals the object’s weight, it floats.
- If the object is denser, its weight is greater than the upthrust, so it sinks.
Key comparison:
If \( \rho_{\text{object}} < \rho_{\text{fluid}} \Rightarrow \) floats
If \( \rho_{\text{object}} > \rho_{\text{fluid}} \Rightarrow \) sinks
Example:
The density of water is \( 1.0~\text{g/cm}^3 \). A plastic cube has a density of \( 0.85~\text{g/cm}^3 \). Will it float?
▶️ Answer/Explanation
Since \( 0.85 < 1.0 \), the cube is less dense than water.
It will float.
Example:
A metal object has a density of \( 7.5~\text{g/cm}^3 \). It is dropped into oil with a density of \( 0.9~\text{g/cm}^3 \). Will it float or sink?
▶️ Answer/Explanation
Since \( 7.5 > 0.9 \), the object is much denser than the oil.
It will sink.
Example:
A submarine adjusts its ballast tanks until its density matches seawater at \( 1.03~\text{g/cm}^3 \). What happens?
▶️ Answer/Explanation
If the submarine’s density equals the fluid, it will neither sink nor float.
This condition is called neutral buoyancy.
Determining Whether One Liquid Will Float on Another
Floating of One Liquid on Another:
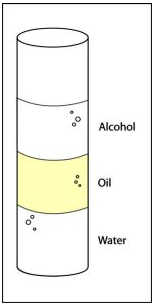
- Whether one liquid floats on another depends on the density of each liquid.
- If the liquids do not mix (are immiscible), the less dense liquid floats on top of the denser one.
Rule:
If \( \rho_{\text{top}} < \rho_{\text{bottom}} \Rightarrow \) top liquid floats
If \( \rho_{\text{top}} > \rho_{\text{bottom}} \Rightarrow \) top liquid sinks (mixes or inverts layer)
Example pairs of immiscible liquids:
- Oil and water
- Alcohol and mercury
- Fuel and water
Example:
Cooking oil has a density of \( 0.91~\text{g/cm}^3 \). Water has a density of \( 1.00~\text{g/cm}^3 \). Which liquid will float?
▶️ Answer/Explanation
Since \( 0.91 < 1.00 \), oil is less dense than water.
Oil will float on water.
Example:
Alcohol has a density of \( 0.79~\text{g/cm}^3 \). Mercury has a density of \( 13.6~\text{g/cm}^3 \). What will happen if alcohol is gently poured over mercury?
▶️ Answer/Explanation
Since alcohol is much less dense than mercury, it will float on top.
Alcohol will float above mercury.
Example:
Fuel has a density of \( 0.80~\text{g/cm}^3 \). Water has a density of \( 1.00~\text{g/cm}^3 \). Will fuel float on water?
▶️ Answer/Explanation
Fuel is less dense than water and they do not mix.
Fuel will float on water.
