CIE IGCSE Physics (0625) Gases and the absolute scale of temperature Study Notes - New Syllabus
CIE IGCSE Physics (0625) Gases and the absolute scale of temperature Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of Gases and the absolute scale of temperature
Key Concepts:
- Effect on Gas Pressure in Terms of Particles
- Temperature Conversion: Celsius and Kelvin
Effect on Gas Pressure in Terms of Particles
Effect on Gas Pressure in Terms of Particles
(a) Change of Temperature at Constant Volume
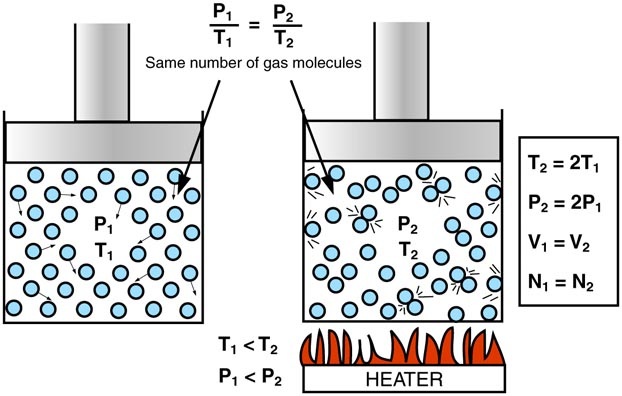
According to the kinetic theory, gas particles are in constant, random motion and exert pressure by colliding with the walls of their container.
When the temperature increases/ while the volume remains constant:
- The average kinetic energy of gas particles increases because temperature is directly proportional to kinetic energy: \( E_k \propto T \)
- Particles move faster and strike the walls more frequently and with greater force.
- This leads to a greater total force exerted per unit area (i.e., higher pressure).
When the temperature decreases at constant volume:
- The average kinetic energy of the gas particles decreases.
- Particles move more slowly, collide with the walls less often and with less force.
- This causes the pressure to decrease.
This relationship is described by Gay-Lussac’s Law (constant volume):
$ \frac{P_1}{T_1} = \frac{P_2}{T_2} $
Conclusion: At constant volume, pressure increases with temperature and decreases when temperature falls.
(b) Change of Volume at Constant Temperature
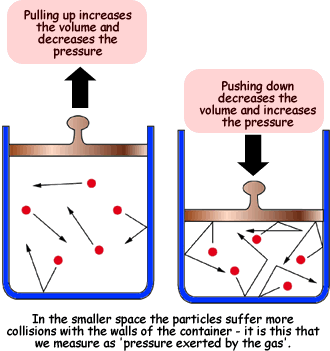
At constant temperature, the kinetic energy of gas particles remains unchanged.
When the volume increases:
- Gas particles have more space to move around.
- They travel longer distances before hitting the walls.
- The frequency of collisions with the walls decreases.
- This results in a decrease in pressure.
When the volume decreases:
- Gas particles are confined to a smaller space.
- They hit the container walls more frequently.
- This results in an increase in pressure.
This relationship is described by Boyle’s Law:
\( P \propto \frac{1}{V} \quad \text{(at constant temperature)} \)
Conclusion: At constant temperature, pressure decreases when volume increases, and increases when volume decreases.
Boyle’s Law:
Boyle’s Law states that for a fixed mass of gas kept at constant temperature, the pressure of the gas is inversely proportional to its volume.
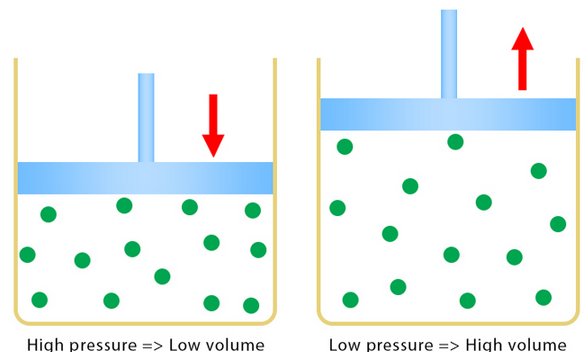
- Mathematically:
\( p \propto \frac{1}{V} \quad \text{(at constant temperature)} \)
- This means:
\( pV = \text{constant} \)
- If pressure increases, volume decreases; if volume increases, pressure decreases — as long as temperature remains constant.
- This law is derived from the kinetic theory of gases, assuming ideal behavior.
Using the equation:
If a gas changes from pressure \( p_1 \), volume \( V_1 \) to new conditions \( p_2 \), \( V_2 \) at constant temperature, then:
\( p_1 V_1 = p_2 V_2 \)
Graphical Representations:
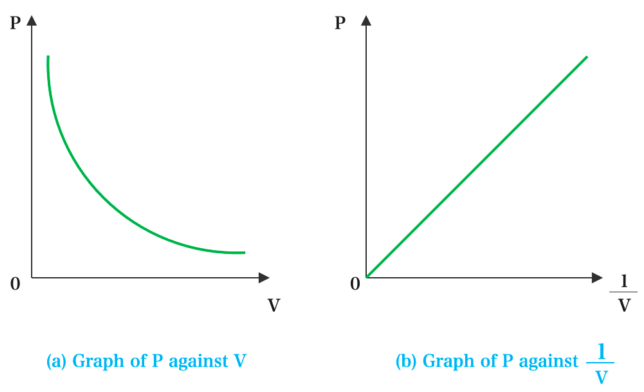
- Graph 1: Pressure \( p \) vs. Volume \( V \)
- This is a curve (hyperbola).
- It shows inverse proportionality: as \( V \) increases, \( p \) decreases.
- Graph 2: Pressure \( p \) vs. \( \frac{1}{V} \)
- This is a straight line through the origin.
- It shows a direct proportionality between pressure and inverse volume.
Example:
Describe qualitatively, in terms of particles, the effect on the pressure of a fixed mass of gas of:
- (a) a change of temperature at constant volume
- (b) a change of volume at constant temperature
▶️ Answer/Explanation
(a) Temperature change at constant volume:
According to kinetic theory, gas pressure is caused by particles colliding with container walls.
- Increasing temperature increases the average kinetic energy of particles.
- Particles move faster and collide more frequently and forcefully with the walls.
- This increases the pressure, as pressure is the force per unit area due to collisions.
- At constant volume, there is no extra space for particles to spread out, so collisions become more intense.
\(\Rightarrow\) Pressure increases with temperature at constant volume.
(b) Volume change at constant temperature:
At constant temperature, the kinetic energy of particles remains constant.
- Increasing volume means particles have more space to move.
- Particles take longer to reach and collide with the walls.
- This decreases the frequency of collisions per second with the walls.
- Fewer collisions mean reduced pressure.
Mathematically, pressure is inversely proportional to volume at constant temperature: \( \boxed{P \propto \frac{1}{V}} \)
\(\Rightarrow\) Pressure decreases with increased volume at constant temperature.
Example:
A fixed mass of gas is kept in a sealed container of constant volume. The gas is initially at a temperature of \( 300\, \text{K} \) and a pressure of \( 1.0\, \text{atm} \). The temperature is then increased to \( 450\, \text{K} \).
Calculate the final pressure of the gas, assuming the volume remains constant and the gas behaves ideally.
▶️ Answer/Explanation
Use Gay-Lussac’s Law (constant volume)
$ \frac{P_1}{T_1} = \frac{P_2}{T_2} $
Substitute known values
$ \frac{1.0}{300} = \frac{P_2}{450} $
Solve for \( P_2 \)
$ P_2 = \frac{450 \times 1.0}{300} = 1.5\, \text{atm} $
Example:
A gas has a volume of \( 2.5\, \text{L} \) at a pressure of \( 100\, \text{kPa} \). If the gas is compressed to a volume of \( 1.0\, \text{L} \) at constant temperature, calculate the new pressure.
▶️ Answer/Explanation
Use Boyle’s Law:
\( P_1 V_1 = P_2 V_2 \)
Substitute the known values:
\( 100 \times 2.5 = P_2 \times 1.0 \)
Solve for \( P_2 \):
\( P_2 = \frac{100 \times 2.5}{1.0} = 250\, \text{kPa} \)
Temperature Conversion: Celsius and Kelvin
Temperature Conversion: Celsius and Kelvin
Temperature in physics is often measured in the absolute scale: the Kelvin (K) scale.
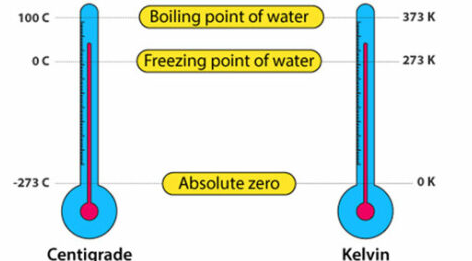
- The Kelvin scale starts at absolute zero, the lowest possible temperature where particle motion stops.
- Absolute zero is equal to \( -273^\circ \text{C} \).
- The size of 1 Kelvin is the same as 1 degree Celsius.
Conversion Formula:
\( T\,(\text{K}) = \theta\,(^\circ \text{C}) + 273 \)
\( \theta\,(^\circ \text{C}) = T\,(\text{K}) – 273 \)
- All thermodynamic calculations (e.g. gas laws, kinetic energy) require temperature in Kelvin.
- You cannot use Celsius in formulas like \( p/T \), \( E_k \propto T \), etc.
- Always convert before using the temperature in formulas.
Example:
Convert \( 25^\circ \text{C} \) to Kelvin.
▶️ Answer/Explanation
Use the formula: \( T = \theta + 273 \)
\( T = 25 + 273 = 298\, \text{K} \)
\( \boxed{T = 298\, \text{K}} \)
Example:
Convert \( 310\, \text{K} \) to degrees Celsius.
▶️ Answer/Explanation
Use the formula: \( \theta = T – 273 \)
\( \theta = 310 – 273 = 37^\circ \text{C} \)
\( \boxed{\theta = 37^\circ \text{C}} \)
| Temperature Conversion Formulas | |
|---|---|
| Equation | Conversion Type |
| \( t_C = \frac{5}{9}(t_F – 32) \) | Convert Fahrenheit to Celsius |
| \( t_F = \frac{9}{5}t_C + 32 \) | Convert Celsius to Fahrenheit |
| \( t_K = t_C + 273.15 \) | Convert Celsius to Kelvin |
| \( t_C = t_K – 273.15 \) | Convert Kelvin to Celsius |
Example:
At a weather station, the outdoor temperature is recorded as \( 95^\circ \text{F} \).
- a. Convert this temperature to degrees Celsius.
- b. Convert the result to Kelvin.
▶️ Answer/Explanation
a. Convert Fahrenheit to Celsius
Use the formula: \( t_C = \frac{5}{9}(t_F – 32) \)
\( t_C = \frac{5}{9}(95 – 32) = \frac{5}{9} \times 63 = 35^\circ \text{C} \)
\( \boxed{t_C = 35^\circ \text{C}} \)
b. Convert Celsius to Kelvin
Use the formula: \( t_K = t_C + 273.15 \)
\( t_K = 35 + 273.15 = 308.15\, \text{K} \)
\( \boxed{t_K = 308.15\, \text{K}} \)
